1. INTRODUCTION
The incidence of diabetes is increasing globally, reaching epidemic levels in low- and middle-income countries, which creates concern in the health care systems with limited resources and persistent challenges in treating communicable diseases (1). Diabetes mellitus is a major health and socioeconomic burden worldwide. Over the past few decades, the alarming increase in its incidence. The prevalence of type 2 diabetes mellitus has tripled in the past 3 decades and is expected to cross more tan 320 million by 2025 (2).
Diabetes mellitus is a heterogeneous metabolic disorder characterized by the presence of hyperglycemia due to impairment of insulin secretion, defective insulin action or both. The chronic hyperglycemia of diabetes is associated with relatively specific longterm microvascular complications affecting the eyes, kidneys and nerves, as well as an increased risk for cardiovascular disease (CVD) (3).
People with type-2 diabetes usually develop the condition after age 45, and the risk for getting it increases with age (4).
Uncontrolled diabetes contributes to the development of neuropathy and peripheral arterial disease by complex metabolic pathways. Loss of sensation caused by peripheral neuropathy, ischaemia due to peripheral arterial disease, or a combination of these may lead to foot ulcers (5). Diabetic foot is a severe chronic diabetic complication that consists of lesions in the deep tissues associated with neurological disorders and peripheral vascular disease in the lower limbs. The incidence of diabetic foot has increased due to the worldwide prevalence of diabetes mellitus and the prolonged life expectancy of diabetic patients (6).
Patients with diabetes are susceptible to infection related to immunodeficiency, neuropathy, and arteriopathy. A significant reduction in bactericidal capacity and phagocytosis may lead to dreaded complications. An infected foot ulcer accounts for ∼60% of lower extremity amputations, making infection perhaps the main proximate basis of this tragic outcome. In a large prospective study of patients with DFU, the existence o infection augmented the risk of a minor amputation by 50% compared to ulcer patients without infection (7).
The most common microorganisms isolated from patients with DFI were reported as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Streptococcus spp., Enterococcus spp., Proteus mirabilis and anaerobes (8).
Although the immunosuppression state associated with diabetes is aknown risk factor for staphylococcal infections, the influence of diabetes in the development of MRSA infection (9). Staphylococcus aureus is the most common causative agent in DFIs, and among these 23.7% were reported as Methicillin-resistant Staphylococcus aureus (MRSA) (10). The most important mechanism of the propagation of MRSA and the other microorganisms is contact between people. The most common transfer is from one person to another by means of contaminated hands of healthcare/auxiliary personnel who do not wash them correctly between one patient and the next. Transmission because of contamination of healthcare material and/or surfaces has also been reported and is known as healthcare-associated infection (HAI) (11).
This pathogen presents many treatment difficulties, particularly in the provision of appropriate empiric antimicrobial therapy. Approximately 40–50% of all S. aureus isolates exhibit methicillin resistance which confirms almost universal beta-lactam resistance (12). Vancomycin, which the antibiotic that was most frequently prescribed, was given in 78% of all antibiotic regimens (13). Knowledge of the prevalence of colonisation or infection of diabetic patients by resistant pathogens, including MRSA, will therefore be important in assessing the extent to which interventions targeted towards diabetic patients may mitigate the spread of resistant pathogens (14).
In the present research work, samples of diabetic foot wounds were collected to first identify S. aureus and the antibiotic resistance patterns shown by methicillin-sensitive strains and, on the other hand, the prevalence of MRSA.
2. METHODS
Diabetic foot wounds were studied in patients between 44 and 84 years of age, with an average age of 59 years, who presented hyperglycemia and different complications associated with diabetic foot at different periods of time. For the present study, exclusion criteria were not considered, since the objective did not involve exclusion or inclusion criteria. With a total of 65 cultures, 37 belong to male patients and 28 to female patients, all living in the Valley of Toluca, Mexico.
For the collection of the sample, it was carried out under adequate hygiene conditions to avoid cross contamination. A sterile swab was used to rub the affected area of the diabetic foot. This swab was placed in Copan transport medium, which allows the growth of aerobic and anaerobic organisms. The medium was stored at room temperature until the primary passage for reseeding. Despite other techniques being preferred in hospital settings, given the nature of the outpatient procedure and the costs of other tests, swab culture was preferred.
For data such as % of glycosylated hemoglobin, specific knowledge of the pharmacological treatment and the explanation of why, are criteria and factors that are not contemplated for the present study.
The primary swab was replated on chromogenic agar, BHI, salt and mannitol, calf blood, EMB and MacConkey, 6 hours after the sample was taken. In addition to the microbial replating, a Gram stain was performed on each of the samples. The Petri dishes were placed in an incubation oven for 18 hours at 37°C.
Once the replatings were obtained, within approximately 18 hours, the strain was identified, as well as the sensitivity to antibiotics with an automated method using the VITEK® 2 equipment. For the tests of tested antibiotics and concentrations, the CLSI m100 standard was followed.To detect resistance to methicillin, the oxacillin 1 µg test and the cefoxitine 30 µg test were used. For the rest of the antibiotics, the following were tested at the indicated concentrations (penicillin 10 µg, ampicillin 10 µg, ampicillin/sulbactam 8/4µg, amoxicillin/clavulanic 10 µg, ceftriaxone 30µg, oxacillin 1 µg, cefoxitin 30 µg, clindamycin µg, erythromycin 15 µg, gentamicin 10 µg, tetracycline 30 µg, levofloxacin 5 µg, ciprofloxacin 5 µg, mofloxacin 5 µg, rifampin 1 µg, trimethoprim/sulfamethoxazole 1.25/23.75 µg, linezolid 4 µg, daptomycin 1 µg).
In this study, the relationship between oxacillin resistance and several clinical factors was analyzed, including glycemic control, erythromycin resistance, arterial hypertension (HTN) and the gender of the patients. To determine the normality of the data, the Kolmogorov-Smirnov and Shapiro-Wilk tests were used and the Spearman correlation coefficient was applied to evaluate the possible relationships between the variables. Statistical analyzes were performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY).
3. RESULTS
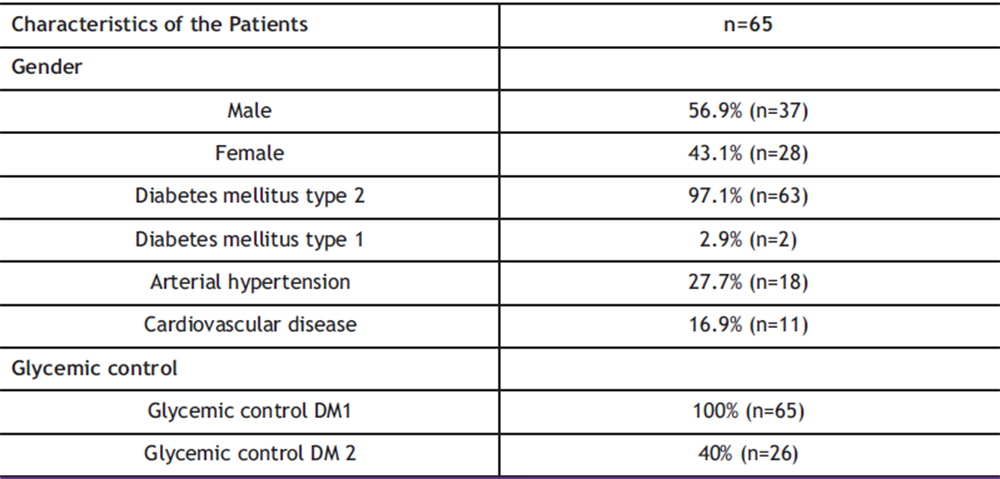
Of the 100% of the sample (n=65), 56.9% (n=37) were men and 43.1% (n=28) were women, of which 97.1% (n=63) had type 2 diabetes mellitus and only 2.9% (n=2) had type 1 diabetes mellitus. Among concomitant diseases, 27.7% (n=18) had additional arterial hypertension, all of them in 100% of people with type 2 diabetes mellitus. For cardiovascular disease, only 16.9% (n=11) were patients with type 2 diabetes. Glycemic control of patients is not something that depends on this study, that follow-up or prescription has been given, however, according to the questionnaire, it was the following: for people with type 1 diabetes mellitus, 100% had control using insulin plus an oral hypoglycemic, prescribed as such by their doctor. treating. For patients with type 2 diabetes mellitus, only 40% (n=26) maintained control. Of the sample, there are patients with controlled DM 2, only 10.7% (n=7) use insulin for glycemic control and 29.3% (n=19) have glycemic control with unspecified oral hypoglycemic agents. Of the entire sample, only 10.7% (n=7) have had more than one reinfection, 1.5% (n=1) of the people who did not present recurrence had MRSA. Table 1 shows the characteristics of the patients in the study.
Table 1. Demographic and Clinical Characteristics of the Patients Studied. This table includes information on patients’ gender, type of diabetes, high blood pressure, cardiovascular disease, and glycemic control.
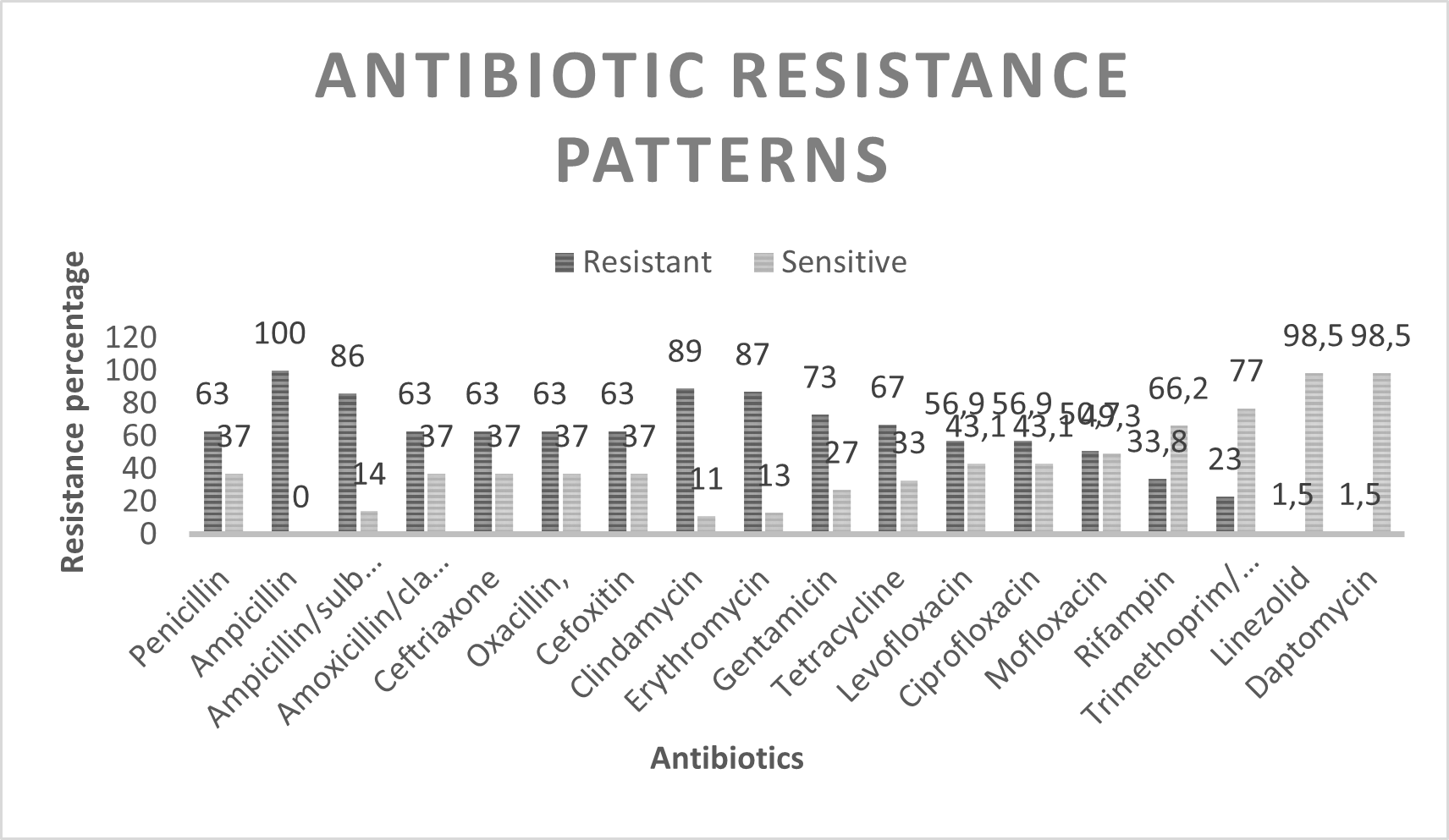
For the antibiotics tested, 100% (n=65) resistance to ampicillin was obtained, 86% (n=56) for ampicillin/sulbactam, which, as described below, suggests the presence of beta-actamases. In order of resistance, clindamycin showed 89% (n=58) resistance and erythromycin 87% (n=57) suggestive of MLS type resistance. For the aminoglycoside gentamicin it was 73% (n=48), tetracycline showed 67% (n=44), in the case of beta-lactams and MRSA markers; penicillin, amoxicillin/clavulanic acid, ceftriaxone and oxacillin, as well as the cefoxitin test, there is 63% (n=41) resistance. The quinolones levofloxacin and ciprofloxacin showed 56.9% (n=37) and mofloxacin 50.7% (n=33), rifampin showed only 33.8% (n=22) and trimethoprim/sulfamethoxazole 23% (n=15), for linezolid and daptomycin alone 1.5% (n=1) showed resistance. Table 2 shows the resistance patterns to the antibiotics tested for S. aureus and Graph No. 1 shows the patterns of resistance and sensitivity to the antibiotics tested. Where a high resistance to beta-lactams is seen, typical of resistance to methicillin in some strains and in others a production of beta-lactamases. On the other hand, it is important to highlight the high MLS-type resistance present, with the erythromycin marker. For quinolones and tetracycline there is also a high resistance of more than 50%.
Table 2: Antibiotic Resistance Patterns in Staphylococcus aureus Isolated from Diabetic Foot Wounds. This table summarizes the percentages of resistance and sensitivity to each antibiotic tested in the study.
Graph 1: Prevalence of Antibiotic Resistance in Staphylococcus aureus Isolated from Diabetic Foot Wounds
4. DISCUSSION
Staphylococcus aureus is one of the most important causes community acquired pathogens. Antibiotic resistance is very common among strains of S. aureus. One of the most resistant forms of this bacterium is methicillin-resistant strains (15). Methicillin is used to treat bacterial infections caused by organisms of the genus Staphylococcus. It is active against certain types of staphylococci, which are resistant to penicillin, but ultimately show resistance to methicillin (16). The high prevalence of strains of S. aureus resistant to methicillin in the community is increasing, as shown in our study, where the population evaluated is small and yet shows 41 strains equivalent to 63% as resistant to methicillin.
Methicillin-resistant Staphylococcus aureus (MRSA) infection was associated with patients in hospitals and skilled nursing facilities. In recent years, reports of community-associated MRSA infections (CA-MRSA) have been increasing. Just 20 years ago in the United States, skin infections caused by methicillin-resistant S. aureus were observed to increase from 29% in 2001 to 64% in 2004 (17).
In a study carried out in 2014 by Lavery and collaborators in the identification of bacteria in diabetic foot, it was shown the prevalence of Staphylococcus aureus was 42.1%, and 70% of these isolates were methicillin resistant (18).
In a study conducted by Lin Shin and collaborators in 2020 in Miaoli, methicillin-resistant Staphylococcus was found in 24.1% of the sample studied, but not a higher percentage of methicillin-sensitive Staphylococcus. In their study they realized that the consumption of oral hypoglycemic agents was a protective factor against Staphylococcus colonization in the nasal passages, but it is not described whether the same occurred in diabetic foot lesions (9). However, in our study, glycemic control by both oral hypoglycemic agents and insulin does not show a correlation that indicates protection against methicillin-resistant Staphylococcus, which is sensitive to methicillin.
In a further study carried out by 250 samples, 48 strains of S. aureus were isolated. Of which 22 presented resistance to methicillin with 45.83%, a percentage similar to that obtained in our study and which suggests the specific prevalence of resistance to methicillin in patients with diabetic foot (19). An important difference is that 100% of the isolates of both coagulase-positive and negative Staphylococcus showed 100% sensitivity to linezolid, a second-line drug when vancomycin also loses effectiveness. In our study, although it is a very low percentage, 1.5% already exists. methicillin-resistant strains with resistance also to linezolid.
In a study in Mexico in 2015 conducted by Estrella and collaborators, in the search for MRSA and its prevalence, in a sample of 100 patients diagnosed with diabetes mellitus, they found a 42% prevalence of S. aureus and of this the 34% showed resistance to methicillin (20). Mario Sanchez in 2017 demonstrated the prevalence of MRSA in patients with diabetic foot infection with a total S. aureus isolate of 67 strains, of which 55% showed resistance to methicillin (21).
Another study conducted in Latin America by Gabriela Carro in 2020 showed a prevalence of S. aureus of 19% of the total isolates, of which 53.8% showed resistance to methicillin,(22) results very similar to those we obtained in our study. The difference is that the methicillin resistance that we present is only from one region of the State of Mexico in Mexico.
On the other hand we could observe a marked MLS (macrolides, lincosamides and streptogramins) type resistance. Three mechanisms are mainly responsible for acquiring resistance to MLS antibiotics in staphylococci: (1) target site modifications by methylation or mutation; (2) active efflux of antibiotics; or (3) inactivation of antibiotics. The first mechanism includes target site modifications by a methylase encoded by one or more of the erm genes, methylating 23S rRNA and thereby altering binding sites for MLS antibiotics (23). In S. aureus is due to the action of efflux pumps, encoded by the mrsA and mrsB genes responsible for pumping macrolide and streptogramin B antibiotics out of the bacteria (MSB resistance phenotype) (24), MLSB phenotype can be expressed into forms of constitutive (cMLSB) or inducible (iMLSB) (25).
In a study conducted by Ortiz and collaborators in 2020 in a hospital in Mexico, they found 100% MLS-type resistance in S. aureus isolates (26).
In our previous study, strains of S. aureus with an MLS-type resistance of 44% and high resistance to fluoroquinolones were identified, this in a tertiary hospital with hospital conversion during the covid 19 pandemic, in 2020.The isolated strains have the classification of MDR and XDR according to the resistance patterns shown (27).
In the statistical análisis the results showed that there is no significant correlation between oxacillin resistance and glycemic control (Rho = -0.043, p = 0.736), erythromycin resistance (Rho = 0.102, p = 0.421), HBP (Rho = -0.096 , p = 0.445) nor gender (Rho = 0.171, p = 0.172).
These findings suggest that oxacillin resistance is not influenced by glycemic control, erythromycin resistance, HTN or gender in the sample studied. The lack of significant correlation could be due to the multifactorial nature of antibiotic resistance, indicating the need to consider other clinical and microbiological factors in future studies. Furthermore, expanding the sample and including additional variables could provide a deeper understanding of the mechanisms underlying oxacillin resistance.
5. CONCLUSION
Diabetes mellitus, especially in low- and middle-income countries, is positioned as a growing threat to global public health. Its impact on health systems is aggravated by the associated complications, among which diabetic foot stands out. This study reveals worrying data: the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) infections in diabetic foot wounds reaches an alarming 63% in the population studied.
This scenario highlights the urgent need to implement effective measures for infection control and the adequate administration of antibiotics in diabetic patients, taking into account that first and second line medications are compromised in a high percentage. The statistical analysis of the study adds complexity to the picture: resistance to oxacillin does not depend solely on glycemic control, high blood pressure, the number of reinfections or gender. This is a multifactorial phenomenon that requires a broader approach, considering both clinical and microbiological factors.
The high resistance observed to multiple classes of antibiotics, including MLS-type resistance and fluoroquinolones, raises alarm bells. It is imperative to develop more robust strategies for the management of infections in patients with diabetes mellitus.
Our study has some limitations, such as the sample size, the lack of molecular studies to detect specific genes present in the isolated strains, among others. However, it shows a clear panorama that gives rise to more in-depth studies.
Financing
This work did not receive funding support.
Conflict of interests
The author declare no conflict of interest.
Acknowledgment
Pasteur Laboratorios for carrying out the laboratory tests.
Code of ethics
The present research work was accepted and approved by the ethics committee of Pasteur Laboratories. On the other hand, the cultures are obtained from a routine sample, therefore, informed concentration is not necessary.
6. REFERENCES
- Kumar A., et al., Prediabetes, undiagnosed diabetes, and diabetes among Mexican adults: findings from the Mexican Health and Aging Study., Annals of Epidemiology., 2016 26, 163e170 https://doi.org/10.1016/j.annepidem.2015.12.006
- Banerjee A., et al., Role of Serum Adiponectin and Vitamin D in Prediabetes and Diabetes Mellitus., Can J Diabetes., 2017, 41(3), 259-265.https://doi.org/10.1016/j.jcjd.2016.10.006
- Punthakee Z., et al., Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome., Can J Diabetes., 2018, 42 S10-S15 DOI:https://doi.org/10.1016/j.jcjd.2017.10.003
- Chou K., Molecular Therapeutic Target for Type-2 Diabetes., J. Proteome Res. 2004, 3, 1284-1288 doi: 10.1021/pr049849v
- Mishra S., et al., Diabetic foot., BMJ. 2017, 359, j5064 Doi:10.1136/bmj.j5064
- Zhang P., et al., Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis., Ann. Med., 2017, 47(2), 106-116 doi: 10.1080/07853890.2016.1231932
- Noor S., et al., Understanding Diabetic Foot Infection and its Management., Diabetes Metab. Syndr., 2017, 11(2), 149-156 https://doi.org/10.1016/j.dsx.2016.06.023
- Saltoglu S., Ergonul O., Tulek N., et al., Influence of multidrug resistant organisms on the outcome of diabetic foot infection., Int J Infect Dis., 2018,70, 10-14 doi: 10.1016/j.ijid.2018.02.013.
- Lin S., et al., Methicillin-resistant Staphylococcus aureus nasal carriage and infection among patientswith diabetic foot ulcer., J Microbiol Immunol Infect., 2020, 53(2), 292-299 https://doi.org/10.1016/j.jmii.2018.03.005
- Viswanathan V., et a., Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infection in India: A Growing Menace., Int. J. Low. Extrem. Wounds.,2019, 18(3) https://doi.org/10.1177/1534734619853668
- Reina-Bueno M., et al., Methicillin-Resistant Staphylococcus aureus Diabetic Foot Crossed Infection: A Case Report., Pathogens., 2020, 9, 549 doi:10.3390/pathogens9070549
- Reveles K., et al., Epidemiology of Methicillin-Resistant Staphylococcus aureus Diabetic Foot Infections in a Large Academic Hospital: Implications for Antimicrobial Stewardship., PLoS ONE, 2016, 11(8), e0161658. doi:10.1371/journal.pone.0161658
- Mergenhagen K., et al., Utility of Methicillin-Resistant Staphylococcus aureus Nares Screening for Patients with a Diabetic Foot Infection., Antimicrob Agents Chemother., 2020, 64(4), e02213-19 doi: 10.1128/AAC.02213-19
- Stacey H., et al., The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: a meta-analysis., Acta Diabetol., 2019, 56, 907-921 https://doi.org/10.1007/s00592-019-01301-0
- Fanaei V., Validi M., Zamanzad B., Karimi A., Isolation and identification of specific bacteriophages against methicillin-resistant Staphylococcus aureus, extended-spectrum beta-lactamases-producing Escherichia coli, extended-spectrum beta-lactamases producing Klebsiella pneumoniae, and multidrug-resistant Acinetobacter baumannii in vitro. FEMS Microbiology Letters, 2021, 368 (19), fnab139 https://doi.org/10.1093/femsle/fnab139
- Al-Hussaniy H., Kadhim Z., Methicillin-Resistant Staphylococcus aureus and New Delhi Metallo beta-lactamases- types of antibiotic resistance, methods of prevention. Med. Pharm. J., 2022, 1(1), 14–24. Med. Pharm. J DOI: 10.55940/medphar20223Med. Pharm. J. DOI: 10.55940/medphar20223Dates
- Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infect Dis. 2005, 11(6), 928-30 doi: 10.3201/eid1106.040641.
- Lavery L., Fontaine J., Bhavan K, Kim P., Williams J., Hunt N., Risk factors for methicillin-resistant Staphylococcus aureus in diabetic foot infections. Diabetic Foot & Ankle, 2014, 5(1) https://doi.org/10.3402/dfa.v5.23575
- Abalkhail A., Elbehiry A., Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections: Protein Profiling, Virulence Determinants, and Antimicrobial Resistance. Applied Sciences. 2022, 12(21), 10803. https://doi.org/10.3390/app122110803
- Cervantes-García E., García-González R., Reséndiz-Albor A., Salazar-Schettino P., Infections of diabetic foot ulcers with methicillin-resistant Staphylococcus aureus. Int J Low Extrem Wounds. 2015, 14(1), 44-9 doi: 10.1177/1534734614564053
- Sánchez-Sánchez M., Cruz-Pulido W., Bladinieres-Cámara E., Alcalá-Durán R., Rivera-Sánchez G., Bocanegra-García V., Bacterial Prevalence and Antibiotic Resistance in Clinical Isolates of Diabetic Foot Ulcers in the Northeast of Tamaulipas, Mexico. Int J Low Extrem Wounds. 2017, 16(2), 129-134 doi:10.1177/1534734617705254.
- Carro G, Saurral R, Salvador Sagüez F, Witman EL. Diabetic Foot Infections: Bacterial Isolates From the Centers and Hospitals of Latin American Countries. Int J Low Extrem Wounds. 2022, 21(4), 562-573 doi:10.1177/1534734620976305
- Li L., Feng W., Zhang Z. et al. Macrolide-lincosamide-streptogramin resistance phenotypes and genotypes of coagulase-positive Staphylococcus aureus and coagulase-negative staphylococcal isolates from bovine mastitis. BMC Vet Res. 2015, 11, 168 https://doi.org/10.1186/s12917-015-0492-8
- Pardo L., Machado V., Cuello D., Aguerrebere P., Seija V., et al., Macrolide-lincosamide-streptogramin B resistance phenotypes and their associated genotypes in Staphylococcus aureus isolates from a tertiary level public hospital of Uruguay. Rev Argent Microbiol,, 2020, 52(3), 202-210 https://doi.org/10.1016/j.ram.2019.10.004
- Khashei R., Malekzadegan Y., Sedigh Ebrahim-Saraie, H. et al. Phenotypic and genotypic characterization of macrolide, lincosamide and streptogramin B resistance among clinical isolates of staphylococci in southwest of Iran. BMC Res Notes . 2018, 11, 711 https://doi.org/10.1186/s13104-018-3817-4
- Ortíz-Gil M., Velazquez-Meza M., Echániz-Aviles G., Mora-Domínguez J., Carnalla-Barajas M., Mendiola Del Moral E., Tracking methicillin-resistant Staphylococcus aureus clones in a hospital in Southern Mexico. Salud Publ Mex., 2022, 62, 186-191.
- Villegas J., Gutiérrez J., Quirino S., Calzonzín P., et al., (2022). Antibiotic resistance patterns in infections associated with health care in a Third Level Center with hospital reconversion in the COVID-19 pandemic. An. R. Acad. Nac. Farm., 2022, 88(2), 123-130.