1. INTRODUCCIÓN
The Baccharis genus contains around 400 species and is found only in the American continent, mainly in Brazil and the Andes. Around 35 species of this genus have been identified in Ecuador, where very few can be found below 1000 meters altitude, with the majority found at 2400 meters altitude, making the species baccharis macrantha a subject of research (1,2).
The baccharis macrantha species is native to Colombia and Ecuador, and is commonly known as “Chilco”, “Chilca”, “Chilco” or “Ciro”, depending on the region where it is found. However, it has been determined that the name “Chilca” corresponds to the Baccharis latifolia species, and “Chilco” to baccharis macrantha (3). In Ecuador, it is found in provinces such as Azuay, Bolívar, Carchi, Cotopaxi, Chimborazo, Imbabura and Tungurahua, and has been used in traditional medicine to treat fungal and bacterial infections, inflammation, menstrual and renal problems, among other ailments, using different preparations such as infusions or poultices, mainly using the leaves (4). It grows vigorously in open terrain with a lot of light, including native shrubs, steep slopes with compact soil, tolerating droughts and extremely cold climates at an altitude of 1800 to 2000 meters above sea level (5).
In 2022, it was evaluated the anti-inflammatory and antioxidant activity of baccharis macrantha species by obtaining the total content of flavonoids and polyphenols, and conducting the evaluation through the red blood cell membrane stabilizer method using only the plant extract. However, a complete phytochemical screening was not carried out for the determination of secondary metabolites. On the other hand, several studies of related species have found secondary metabolites that confer different pharmacological properties, including terpenes, phenols, flavonoids, and coumarins, which are the main metabolites of interest for anti-inflammatory activity (3). In 2008, designed a topical anti-inflammatory formulation based on Baccharis latifolia, which was developed in two main stages: obtaining, identifying, and evaluating the anti-inflammatory activity of the hydroalcoholic extract, and evaluating the anti-inflammatory activity of a topical pharmaceutical form. It was concluded that the pharmaceutical form designed as a gel cream presents a greater anti-inflammatory effect than the effect observed in the hydroalcoholic extract (6).
Since there are not enough studies on the species baccharis macrantha, the aim was to evaluate the anti-inflammatory activity of a gel with different concentrations of ethanol extract from this plant in mice using the carrageenan-induced plantar edema method (7). Anti-inflammatory activity is important because inflammation is a common pathology in society, mainly restricting mobility and dexterity, making it more difficult to perform daily activities, and also aiming to identify the important metabolites in this activity.
2. MATERIALS AND METHODS
2.1. Plant Material
Leaves of the baccharis macrantha plant were collected in the Llucud Alto community in the Chambo canton, because as previously mentioned, this species tends to grow in very cold climates with steep slopes and compact soils, and this community has the ideal characteristics for the growth of this species, as it is located on the slopes of the El Altar volcano, belonging to the province of Chimborazo, Ecuador, at an altitude of approximately 1700 to 2100 meters above sea level, with temperatures of 10 degrees Celsius below zero, considered a cold climate with compact soils, and its collection is difficult during rainy seasons.
2.2. Experimental animals
Thirty-six male mice of the Mus musculus species, weighing approximately between 30 and 45 grams, were used and divided into 6 groups of 6 mice, each group with similar weight. They were subjected to a 12-hour fast to ensure greater absorption during the administration of treatments, and kept at appropriate temperatures in the animal facility of the Faculty of Sciences of the Polytechnic School of Chimborazo for their respective conservation. This species of mice was selected because it is considered a common organism in in vivo experimentation, due to its short generation time, easy breeding, and visible phenotypic characteristics. Therefore, induced inflammation could be observed with the naked eye, making it optimal for measurement. It is worth noting that the guidelines of the National Institutes of Health (NIH Publications No. 8023, revised 1978) were used for animal experimentation.
2.3. Design type
An experimental design was used to evaluate the anti-inflammatory activity of 3 concentrations of gels at 10%, 15%, and 18.75% of the ethanolic extract of baccharis macrantha. These concentrations were chosen because in previous studies with similar species, it was noted that gels above 20% generated adverse reactions and were toxic to experimental animals. Therefore, gels lower than 20% were selected. Additionally, these concentrations were compared with commonly used commercial gels, with Diclofenac at 1% and Naproxen at 5.5% as dependent variables in mice. Carrageenan at 0.5% was induced in the mice using the plantar edema method.
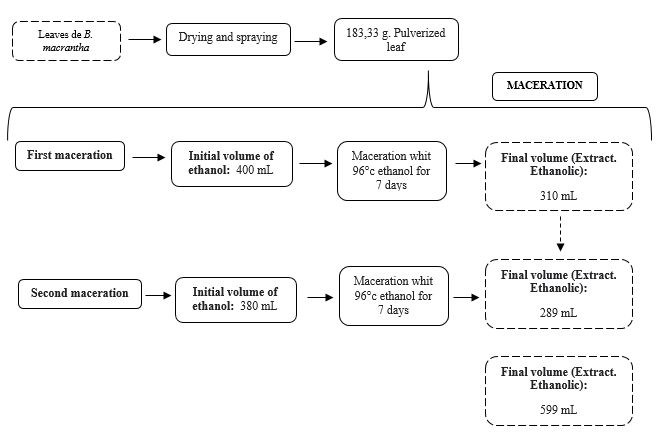
Figure 1. Scheme for the preparation of ethanolic extract
2.4. Preparation of the ethanolic extract
Principio del formulario
The ethanolic extraction was carried out with leaves from the whole and crushed plant, in which a weight of 152,39 grams of dry leaves was obtained, and upon crushing them, a weight of 183,33 grams. Then, extraction by maceration was performed, where the crushed plant leaves were placed in an amber flask of 5000 mL with 400 mL of 96° ethanol; consequently, two macerations were performed, the first for the initial 7 days. For the second maceration, the same leaves were placed, filtered with 380 mL of 96° ethanol for 7 days, filtering in each of the macerations, obtaining a total of 599 mL of ethanolic extract, placing it in another amber flask.
2.5. Fitochemical screening
The phytochemical screening of the ethanol extract was carried out, which included reactions to identify the presence or absence of metabolites such as flavonoids, coumarins, quinones/anthraquinones, alkaloids, triterpenes, saponins, and tannins/phenolic compounds, where the quantification of flavonoids and phenols was also carried out by the “Folin Ciocalteu method” (7), to determine the exact amount of these metabolites in the extract.
2.6. Preparation and quality control of gels
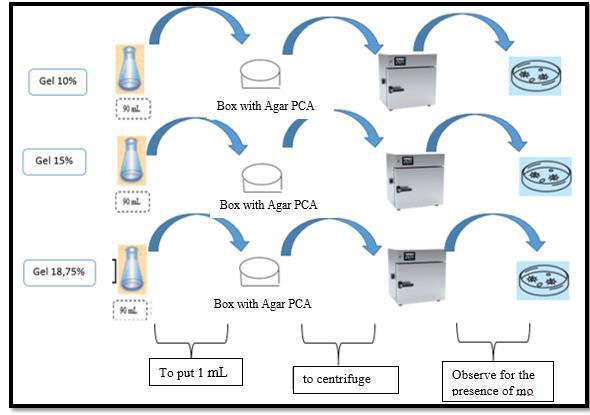
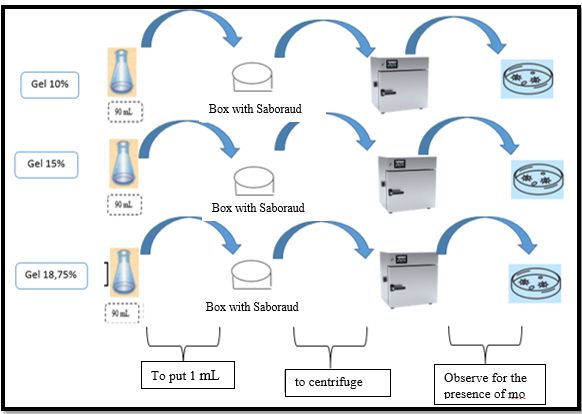
For the formulation and preparation, first, the concentrated ethanol extract was obtained, for which approximately 550 mL of liquid ethanol extract was placed in the flask of the rotary evaporator, the base of the equipment was heated, trying not to take much pressure, until it is seen that the 96° ethanol has been completely distilled, then the extract was removed from the flask using a spatula, placing it in a Petri dish previously weighed without a sample, from which approximately 4g of concentrated ethanol extract was obtained. Therefore, the 3 gels were prepared, where first, 4g of Carbopol 940 was dissolved in 1000 mL of distilled water with stirring by means of the magnetic stirrer, in a 1000 mL beaker. Next, the beaker was placed in the gel mixer, and depending on the formulation, the mL of glycerin was added with constant agitation, by means of the gel mixer, then the mL of Triethanolamine (TEA) was added depending on its formulation with slow movements. Finally, the concentrated extract of baccharis macrantha was incorporated into the mixture in respective concentrations (10%, 15%, and 18.75%), it was stirred with the help of the gel mixer until the mixture was uniform, and then it was packaged and labeled. Finally, quality control was performed, determining physicochemical characteristics (pH, greasiness, viscosity, and stability) and microbiological control such as total coliforms described in Figure 2, mesophilic aerobes as shown in Figure 3, and fungi and yeasts in Figure 4.
2.7. Evaluation of the anti-inflammatory activity of the gels
For the evaluation of the anti-inflammatory activity, 36 mice were handled under the conditions mentioned above. Afterwards, 6 mice with similar weight were divided into 6 different groups: the first group called “control group”, whose mice were only injected with carrageenan, the “positive groups” being the commercial gels, and finally the remaining 3 groups belonging to the gel with 10%, 15% and 18.75% ethanol extract, respectively. Then, the mice were labeled with a marker in some part of their tails to correctly distinguish them at the time of application of the treatments or gels. Therefore, the different treatments were administered to the mice through the method of carrageenan-induced plantar edema at 0.5%, which is based on subcutaneously injecting a 0.5% carrageenan solution in the mouse’s paw, and the times for data collection were determined. The first time was at the moment carrageenan was placed (0’), then measurements were taken at 30 minutes after the carrageenan application (30’), at which point the treatment was applied, either the Positive group treatment (1% diclofenac and 5.5% naproxen) or the gels (10%, 15%, and 18.75%). The swelling was then evaluated every hour for 6 hours using a caliper that provides millimetric measurements, and finally the data obtained from the 6 groups was tabulated and replaced in the formula to calculate the percentage of inflammation. For the statistical analysis, the MINITAB version 2019 program was used, using the one-way ANOVA test, where the p-value was taken into account. Thus, if p ≥ 0.05, the null hypothesis (H0) is accepted and it is concluded that there is no significant difference between the treatments. However, if p < 0.05, the alternative hypothesis (H1) is accepted, and the Tukey test is performed to determine where there may be a significant difference between all groups or treatments.
Figure 2. Scheme for total coliform count
Figure 3. Diagram for counting mesophilic aerobes
Figure 4. Diagram for counting fungi and yeasts
3. RESULTS
3.1. Phytochemical screening
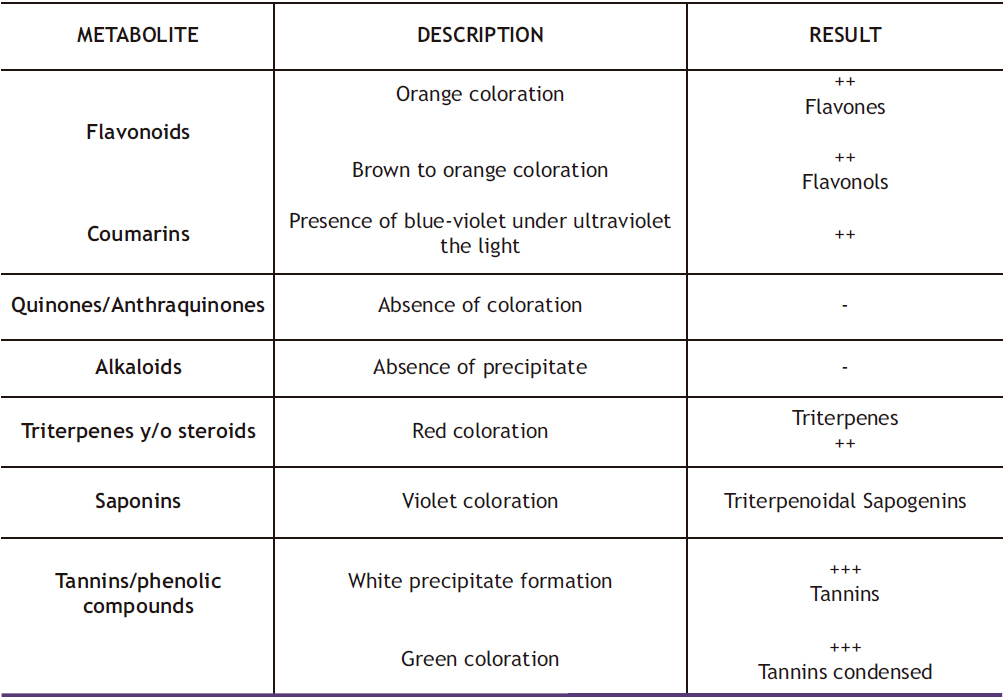
In the respective phytochemical screening of the ethanol extract of the leaves of the baccharis macrantha plant presented in Table 1, the presence of flavonoids such as flavones and flavonols, saponins, tannins, and triterpenes was indicated due to the presence of different colorations for each assay. Additionally, saponins showed violet coloration for triterpenoidal saponins, and condensed tannins showed green coloration, both of which are important in the anti-inflammatory activity. The presence of coumarins was also determined through an ultraviolet light chamber. Regarding the quantification of phenols, it was observed that the total phenol content present in the baccharis macrantha leaves was 113 mg equivalents of gallic acid/g of dry extract, which is 11.30 g equivalents of gallic acid/100 g of dry extract. As for the quantification of total flavonoids, it was 53 mg equivalents of gallic acid/g of dry extract, that is, 5.3 g equivalents of gallic acid/100 g of dry extract.
Table 1. Phytochemical screening of the ethanol extract of the baccharis macrantha plant.
3.2. Elaboration and quality control of gels
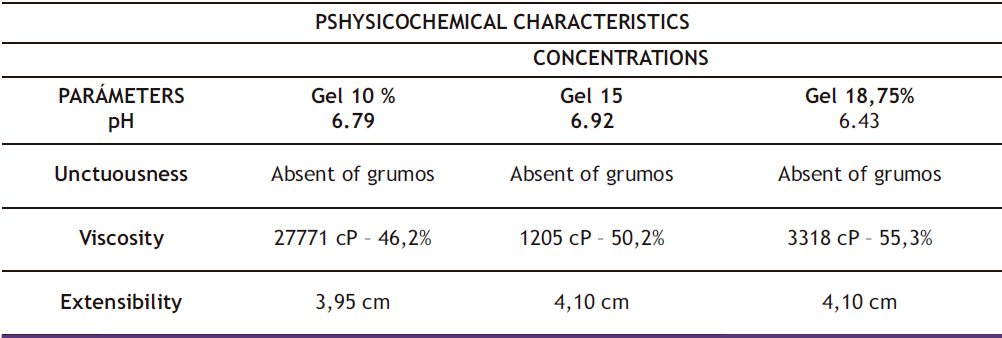
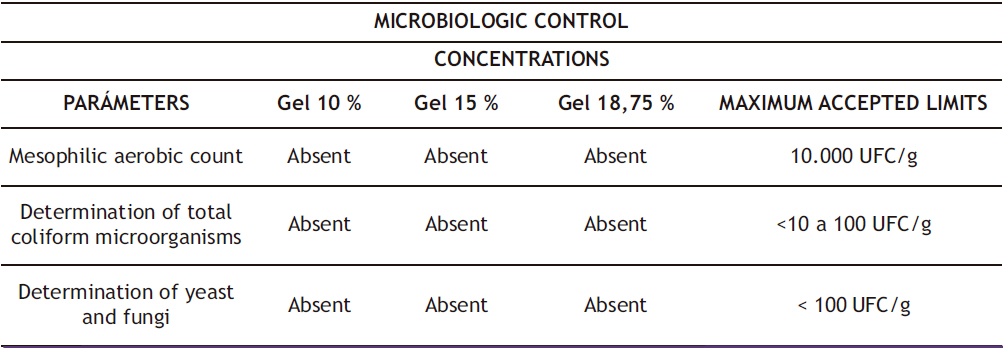
During the quality control of the gel formulations, their physicochemical characteristics were determined and presented in Table 2. It was observed that they have an acidic pH, with the 10% gel having a pH of 6.79, the 15% gel 6.92, and the 18.75% gel 6.43. Smooth consistency was determined by absence of lumps or any foreign objects during tactile assessment of unctuosity. Viscosity of the three gels was found to be proportional to their concentration. The three concentrations were found to have similar extensibility, and no foreign matter was observed. Microbiological control revealed absence of mesophilic aerobes, total coliforms, fungi, and yeast, as shown in Table 3 for the 10%, 15%, and 18.75% concentrations respectively, indicating the safety of the manufactured gels.
Table 2. Physicochemical Characteristics of Gels
Table 3. Microbiological control of gels
3.3. Anti-inflammatory evaluation
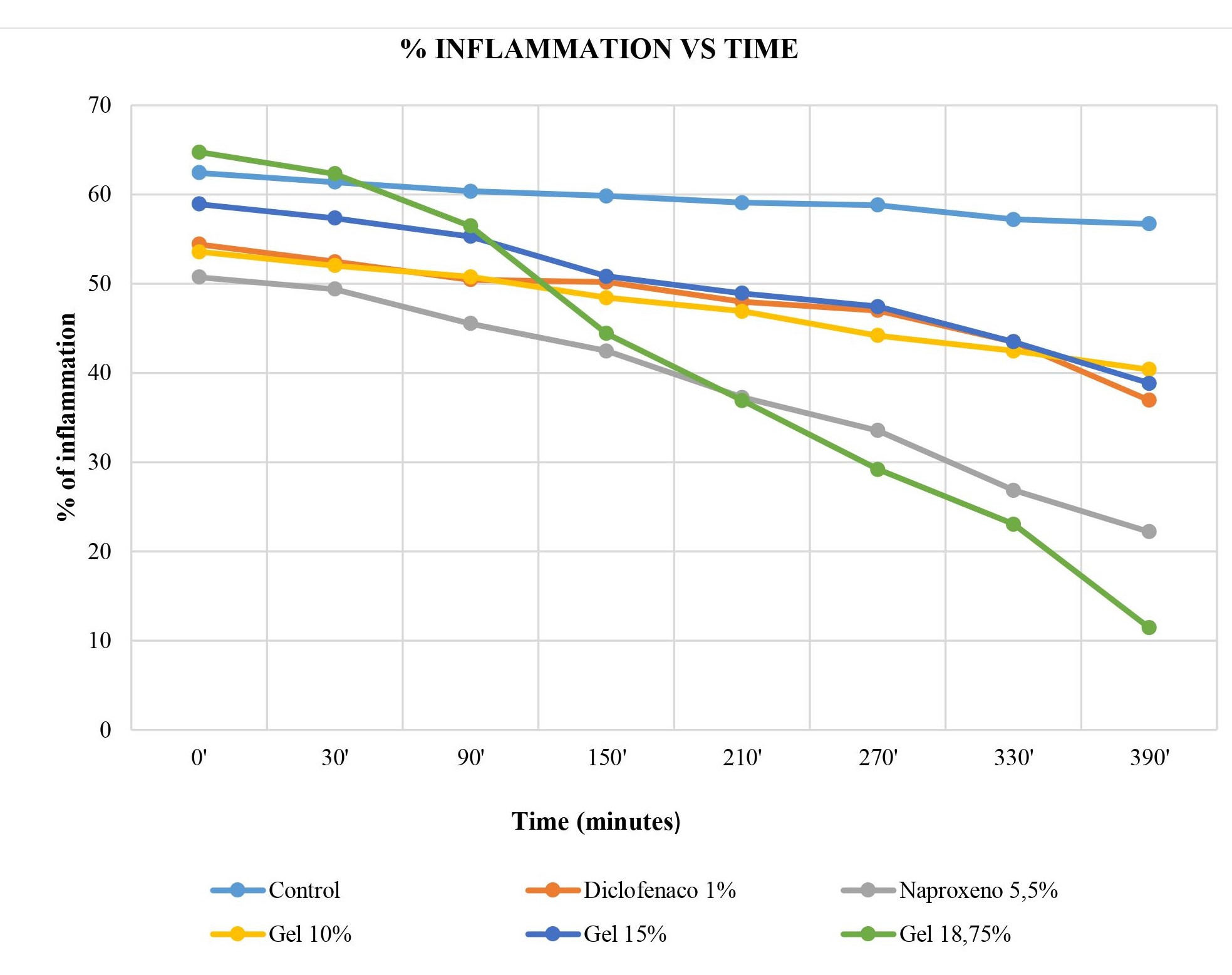
When conducting the anti-inflammatory evaluation, we noted several important aspects, such as the times at which noticeable inflammation reduction occurred, as well as the anti-inflammatory percentage, as shown in Figure 5. We observed that there was greater inflammation reduction in the group that used the 18.75% gel, as after 40 minutes of carrageenan and gel application, there was already inflammation reduction in the mouse paw. However, we also noted that the positive control group (5.5% Naproxen) had a similar anti-inflammatory effect, as there was a decrease in the percentage of inflammation after 50 minutes of treatment. It is worth noting that the other baccharis macrantha-based gels at 10% and 15% also had mild anti-inflammatory activity, but it took longer to visualize their inflammation reduction.
Figure 5. Comparison of the percentage of inflammation vs time
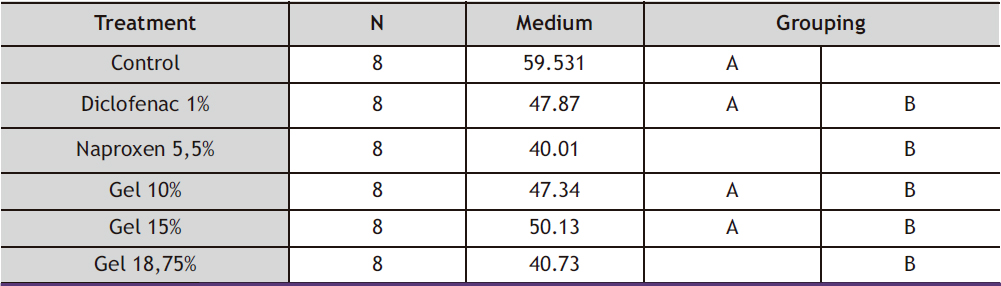
Table 4. ANOVA statistical analysis of the inflammation percentages of the groups in the anti-inflammation evaluation
Table 5. Tukey Test analysis of inflammation percentages of groups in anti-inflammatory evaluation
3.4. Statistical analysis
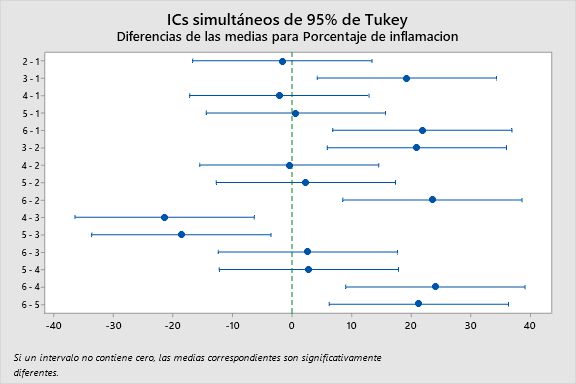
When performing the statistical analysis using analysis of variance (ANOVA) with a 95% confidence level as shown in Table 4, the p-value obtained was 0.002, which means that p<0.05, thus rejecting the null hypothesis and accepting the alternative hypothesis. Therefore, it was concluded that one of the treatment group means was different, and a Tukey test was performed with a 95% confidence level as shown in Table 5. It was found that the 18.75% gel and 5.5% Naproxen had a significant difference, meaning that these groups had higher anti-inflammatory activity than the 1% Diclofenac and the other gels based on the ethanol extract of baccharis macrantha. The 18.75% gel was the maximum concentration used, and the fact that the group doesn’t share a letter means that it shows a significant difference as shown in Table 4. For verification purposes, a simultaneous Tukey’s 95% confidence interval plot was also created, where the difference was visualized better through whiskers. If the whisker does not contain zero, it means that they are significantly different, which confirms that the 18.75% gel and 5.5% Naproxen have higher anti-inflammatory activity, as shown in Figure 6.
Figure 6. Simultaneous Tukey confidence intervals of inflammation percentages of treatment groups in anti-inflammatory evaluation
4. DISCUSSION
As there were no previous investigations on phytochemical screening of baccharis macrantha species, the results were compared with other similar species of the same genus (6), on Baccharis latifolia, being like this, several secondary metabolites were detected, with emphasis on the important metabolites for anti-inflammatory activity, such as phenolic compounds, tannins, saponins, and triterpenoid compounds, particularly monoterpenoids, mainly limonene, all of which were found in abundant quantities. In terms of coumarins on Baccharis genistelloides, detected abundant amounts of this metabolite in the ethanol extract, as well as high amounts of tannins. As the same metabolites were detected in baccharis macrantha species in high quantities, it was determined that the species has significant anti-inflammatory activity (9). Regarding the quantification of phenols and flavonoids using the Folin Ciocalteu method, the results showed that this plant species had a higher amount of total phenols and flavonoids, with values of 113 and 53 mg, respectively (1), quantification of phenols in baccharis macrantha determined 80.1 mg EQ/g of dry extract and 21 mg EQ/g of dry extract for flavonoids. Regarding the physicochemical characteristics of the gels, they had an acidic pH within the established range (10), which states that gels should be within a pH range of 4-7. Regarding viscosity, there is no specific value, as they have their own characteristics. Regarding extensibility, indicated that the value should be a maximum of 5 cm for the average radius, and all concentrations were within normal values (11). For microbiological control is there should be a maximum limit of 10,000 CFU/g for mesophilic aerobes and >100 for fungi and yeasts, and the gels produced were within the required range (12). The absence of total coliforms, mesophilic aerobes, fungi, and yeasts in the Baccharis latifolia gel (13).
In the study of Baccharis, the highest concentration formulation (7 mg/g or 7%) showed a greater anti-inflammatory effect, and statistical analysis using ANOVA indicated a significant difference, with the 7% gel showing greater anti-inflammatory activity than the other treatments. It had the same anti-inflammatory power as its control group. Therefore, both species, Baccharis latifolia and baccharis macrantha, have anti-inflammatory activity, with different concentrations, but still possessing anti-inflammatory properties. Also evaluated the anti-inflammatory activity of the same species, but using the red blood cell stabilization method with only the ethanol extract (3).
5. CONCLUSIONS
In the present research, important metabolites were identified in the anti-inflammatory activity such as flavonoids, tannins, saponins, and coumarins, which are crucial for the anti-inflammatory property and are of great importance for the evaluation of various important pharmacological activities for the treatment of other pathologies, since there are no studies that determine such activity with the ethanolic extract of baccharis macrantha, making it innovative and interesting to conduct more research on the treated species. Gels based on the leaves of the ethanolic extract of baccharis macrantha showed anti-inflammatory activity when evaluated in mice, using the plantar edema method induced by 0.5% carrageenan. Regarding the three gels formulated, it was demonstrated that the gel formulation at 18.75% has a greater anti-inflammatory effect, confirmed by the ANOVA statistical analysis and the Tukey Test, which also complied with all the physicochemical and microbiological quality controls established by the USP. Therefore, by complying with all the parameters and proving its anti-inflammatory activity, the gels could be used as a treatment in humans with acute and possibly severe inflammatory processes, providing quick and effective improvement. It is also recommended to give more importance to developing pharmacological products based on plants, since they are less toxic and rescue ancestral traditions in the use of plants for the treatment of pathologies.
It is funded by the participating authors
7. REFERENCIAS
- Prada J, Ordúz-Díaz LL, Coy-Barrera E. Baccharis latifolia: una Asteraceae poco valorada con potencialidad química y biológica en el neotrópico. Rev Fac Cienc Básicas. 2016;12(1):92–105. Avaible at: (http://dx.doi.org/10.18359/rfcb.1858)
- Andes Tress (página de inicio de Internet). Baccharis L.; 2008. Disponible en: (http://www.efloras.org/florataxon.aspx?flora_id=201&taxon_id=103317)
- Rosero, S., Del Pozo, F., Simbaña, W., Álvarez, M., Quinteros, M. F., Carrillo, W., & Morales, D. Polyphenols and flavonoids composition, anti-inflammatory and antioxidant properties of andean baccharis macrantha extracts. Plants (Basel). 2022; 11(12), 1555. https://doi.org/10.3390/plants11121555
- LADERA SUR (página de inicio de Internet). El chilco y sus singulares beneficios para la naturaleza.; 2018. Disponible en: (https://laderasur.com/articulo/el-chilco-y-sus-singulagres-beneficios-para-la-naturaleza/)
- Flickr (página de inicio de Internet). Ciro, camiseto (baccharis macrantha).; 2007. Available at: (https://www.flickr.com/photos/16895199@N04/32591621671/in/photostream)
- Hoyos Vargas, K. M., & Yep Chu, M. Y. Design of a topical application formulation based on Baccharis latifolia (Chilca), with anti-inflammatory effect., Universidad Nacional Mayor de San Marcos (2018) Available at: https://cybertesis.unmsm.edu.pe/handle/20.500.12672/1615
- Vargas Maji, N. Determination of the Anti-inflammatory Activity of Campyloneurum amphostenon by Inhibition of Carrageenan-Induced Plantar Edema in Rattus norvegicus Rats, Escuela Superior Politécnica de Chimborazo (2017). Available at: http://dspace.espoch.edu.ec/bitstream/123456789/6692/1/56T00708.pdf
- Muñoz-Bernal, Ó. A., Torres-Aguirre, G. A., Núñez-Gastélum, J. A., de la Rosa, Laura A., Rodrigo-García, J., Ayala-Zavala, J. F., & Álvarez-Parrilla, E. Nuevo acercamiento a la interacción del reactivo de folin-ciocalteu con azúcares durante la cuantificación de polifenoles totales. TIP. Revista Especializada en Ciencias Químico-Biológicas, 20(2), 23-28, (2017). Avaible at: (https://doi.org/10.1016/j.recqb.2017.04.003)
- Herrera Fuentes, Ingrid A., Quimis Ponce, Katty L., Sorroza Rojas, Nancy A., García Larreta, Frella S., Mariscal, Walter, Sorroza Rojas, Nancy A., & Mariscal García, Raisa S. (2017). Determinación de Taninos y Cumarinas presente en la planta tres filos. Polo del Conocimiento. 2017; 2(7). https://doi.org/10.23857/pc.v2i7.257
- United States Pharmacopeia Convention USP XXV. 25ed. Rockville. US. United States Pharmacopeial Convention, Inc. 2002.
- United States Pharmacopeia Convention USP XXVIII. 28ed. Rockville. US. United States Pharmacopeial Convention, Inc. 2005.
- United States Pharmacopeia Convention USP XXIX. 29ed. (Microbial Limit. United States Pharmacopeial convention. 2006.
- Aragadvay Yungan, Sandra Piedad. Elaboration and quality control of dye and healing and anti-inflammatory gel based on chilca (Baccharis latifolia) and Hierbamora (Solanum nigrum). [Undergraduate thesis, Escuela Superior Politécnica de Chimborazo (2009). Available at: http://dspace.espoch.edu.ec/bitstream/123456789/216/1/56T00190.pdf