The increasing prevalence of obesity among children and adolescent’s wide is a public health problem, resulting from the interaction of genetic, environmental and lifestyle factors. Obesity can lead to dysbiosis of the gut microbiota. This systematic review aims to gather scientific information available on the composition of gut microbiota in children/ adolescents with overweight/obesity. Research studies were identified through a scientific database (PubMed). The key words used were “Obese” OR “Overweight” AND “adolescent” OR “children” AND “microbiota”. Observational and intervention studies in children/adolescents having either overweight or obesity were included in this review, belonging to the last ten years – from December 2012 to October 2022. The initial search resulted in 409 references, 379 of them were excluded because the participants had major pathologies other than obesity or overweight. From the remaining articles, others were excluded due to not providing information on the number of participants, or not including data on microbiota composition. A total of 16 articles were selected: 12 observational studies and 4 intervention studies. Among the observational studies that compared overweight/obesity vs. normal weight or metabolically unhealthy obese vs. metabolically healthy obese children/adolescents, at least two studies found higher levels of Firmicutes, Proteobacteria, Bacteroidales, Adlercreutzia, Bifidobacterium, Escherichia coli, and Clostridium. Moreover, lower abundances of Bacteroidetes, Verrucomicrobia, Bacteroides, and Akkermansia were observed. Regarding intervention studies consisting of supplementation of oligofructose- enriched inulin and a weight reduction program, higher proportions of Actinobacteria were observed after the intervention. Clostridia was also found in higher abundances after interventions that used a combined strength and endurance training program and a weight reduction program. The findings suggest that obesity decreased microbiota diversity and increases species associated with inflammation. The results are consistent with previous studies in adults. This information will be useful for designing dietary interventions to prevent or reverse dysbiosis in individuals with obesity.
Keywords: Obese; Overweight; Adolescents; Children; Microbiota; Intervention studies; Observational studies
RESUMEN:
La creciente prevalencia de obesidad en niños y adolescentes es un problema de salud pública, resultado de la interacción de factores genéticos, ambientales y de estilo de vida. La obesidad puede provocar una disbiosis de la microbiota intestinal. Esta revisión sistemática tiene como objetivo recopilar información científica disponible sobre la composición de la microbiota intestinal en niños/adolescentes con sobrepeso/obesidad. Los estudios de investigación se identificaron a través de una base de datos científica (PubMed). Las palabras clave utilizadas fueron “obeso” O “Sobrepeso” Y “adolescente” O “niños” Y “microbiota”. En esta revisión se incluyeron estudios observacionales y de intervención en niños/adolescentes con sobrepeso u obesidad, pertenecientes a los últimos diez años, de diciembre de 2012 a octubre de 2022. La búsqueda inicial resultó en 409 referencias, de las cuales 379 fueron excluidas porque los participantes tenían patologías mayores además de la obesidad o el sobrepeso. De los artículos restantes, se excluyeron otros por no proporcionar información sobre el número de participantes o por no incluir datos sobre la composición de la microbiota. Se seleccionaron un total de 16 artículos: 12 estudios observacionales y 4 estudios de intervención. Entre los estudios observacionales que compararon el sobrepeso/obesidad frente al peso normal o los niños y adolescentes obesos metabólicamente no saludables frente a los obesos metabólicamente sanos, al menos dos estudios encontraron niveles más altos de Firmicutes, Proteobacterias, Bacteroidales, Adlercreutzia, Bifidobacterium, Escherichia coli y Clostridium. Además, se observaron menores abundancias de Bacteroidetes, Verrucomicrobia, Bacteroides y Akkermansia. En cuanto a los estudios de intervención consistentes en suplementación con inulina enriquecida con oligofructosa y un programa de reducción de peso, se observaron mayores proporciones de Actinobacteria después de la intervención. Los clostridios también se encontraron en mayor abundancia después de las intervenciones que utilizaron un programa combinado de entrenamiento de fuerza y resistencia y un programa de reducción de peso. Los hallazgos sugieren que la obesidad disminuye la diversidad de la microbiota y aumenta las especies asociadas con la inflamación. Los resultados son consistentes con estudios previos en adultos. Esta información será útil para diseñar intervenciones dietéticas que prevengan o reviertan la disbiosis en individuos con obesidad.
Palabras clave: Obesidad; Sobrepeso; Adolescentes; Niños; Microbiota; Estudios de intervención; Estudios observacionales
1. INTRODUCTION
1.1. Gut microbiota
The human microbiome consists of bacteria, viruses, fungi, protozoa, and archaea that colonize the gastrointestinal tract, as well as other parts of the body such as airways and skin. From the first days of life, it gradually matures and develops accordingly to individual growth (1). Currently, there are more than 1000 defined species that can be grouped into five predominant phyla: Firmicutes, Bacteroidetes, Verrucomicrobia, Actinobacteria and Proteobacteria (2).
Postnatal intestinal colonization is determined by complex factors, some of which include the mother’s diet during pregnancy and lactation, the type of delivery, early skin-to-skin contact, weaning period, use of antibiotics during infancy, environment, and family members living in the same household (3,4). The colonization of the neonate by microbes is the beginning of a lifelong human-microbe symbiosis (5). While human-specific gut microbiome composition can differ, the variation itself is considered physiological in the context of a healthy gut microbiota according to age, sex, ethnicity, climate, and lifestyle -dietary- habits, among others (2).
The human microbiota helps maintain health and well-being. More importantly, our microbes have metabolic activities and can synthetize important metabolites that can have an effect in the host (6). They participate in the control of our metabolism, how we digest and store nutrients. They can metabolize bile salts which affect our fat metabolism, synthesize vitamin K which helps in coagulation, degrade protein to amino acids, ferment polysaccharides which we cannot digest, and produce short chain fatty acids (SCFA) (6). Some of them, such as butyrate and propionate, can provide energy to epithelial cells and induce satiety (2). Moreover, there is evidence that our intestinal bacteria are capable of producing serotonin, melatonin, acetylcholine, and gamma-aminobutyric acid (GABA) which affect appetite regulation or body weight (6).
In obesity (OB) and its associated comorbidities, the composition of gut microbiota and intestinal epithelial barrier functions are altered, which is known as “dysbiosis” (7–10). Dysbiosis can be defined as a decrease in microbial diversity, reduced beneficial bacteria, and an increase in proinflammatory species (bacteria that become pathogenic under certain conditions) (10). While there is no clear understanding of a “healthy” colonic microbiota, several gut microbiota species may be associated with OB (11). Specifically, several gut microbiota metabolites could participate in the induction of a low-grade chronic inflammation (4,12,13). Targeting gut microbiota composition through dietary interventions can be considered a therapy option for reversing dysbiosis in patients with OB caused by diet.
1.2. Obesity and overweight in pediatric populations
OB is derived from a positive energy imbalance between energy consumed and energy expended maintained over time. It is a disease characterized by excessive fat accumulation and a low -grade chronic inflammation that presents a risk to health (14). Globally there has been an increase in the intake of high-energy-dense foods that are high in sugars and fats, and a decrease in physical activity (15).
Since pediatric populations are continuously growing it is difficult to apply the adult fixed value of 25 or 30 kg/m2 of body mass index (BMI) as the overweight (OW) or OB criteria. The World Health Organization (WHO) recommended the use of BMI-SDS or BMI z-score as a measure of relative adjusted weight for the child’s age and sex. The interpretation cutoffs are as follows: OW: > + 1 SD (equivalent to BMI 25 kg/m2 at 19 years), OB: > 2 SD (equivalent to BMI 30 kg/m2 at 19 years) (16).
In the United States, childhood OB has tripled since 1980 for adolescents and doubled for children (17). In the European Region, according to WHO report, one in three children aged 6-9 years had OW or OB (18). In the study on nutrition, physical activity, child development and OB in Spain (ALADINO) report, the figures for Spain were 23,3% of children with OW and 17,2% with OB (19). According to the Childhood Obesity Surveillance Initiative (COSI), OW and OB affect 29% of children aged 7-9 years old in the European region (20).
Pediatric OB is associated with serious consequences for health and social life and is a global public health challenge due to its relationship with premature death and disability in adulthood (18). Children who are OB are more likely to suffer from glucose intolerance, non-alcoholic fatty liver disease, dyslipidemia, hypertension, and cardiovascular disease (21). Considering that childhood OB persists into adulthood, prevention is essential for amelioration of this global problem. There are a variety of treatments for OB, mainly lifestyle modifications, medication, education, and counseling. Recent studies have proven that family implication, as well as intensive counseling in combination with behavior and lifestyle changes (diet and exercise) are the most effective strategies for weight loss.
The objective of this systematic review is to summarize the evidence on gut microbiota composition in a pediatric population with OW or OB. This knowledge could be useful for designing strategies to prevent or treat microbiota dysbiosis.
2. METHODS
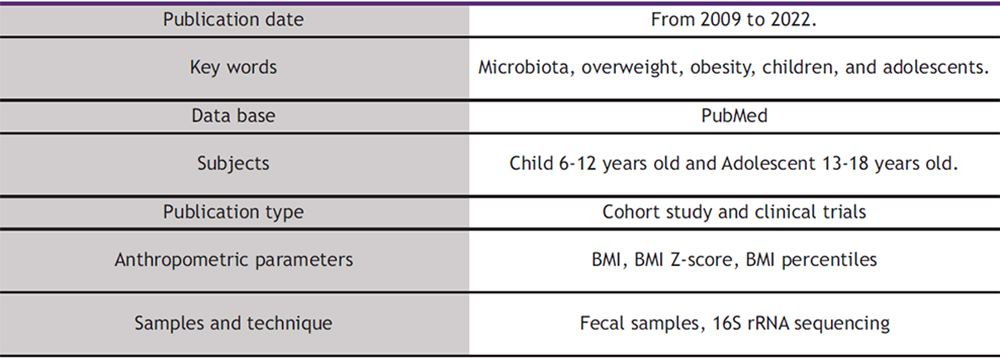
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement criteria for its elaboration. Relevant studies were identified through a search in the scientific database PubMed, and the studies included were published between December 2012 and October 2022. The key words used for the literature search were “Obese” OR “Overweight” AND “adolescent” OR “children” AND “microbiota”. Table 1 illustrates the criteria used to search for the selected articles.
This review included randomized and non-randomized control trials and intervention studies in children/adolescents, with either OW or OB. Studies were excluded if they: a) did not include children/adolescents with OW or OB; b) involved patients diagnosed with diseases such as autism, diabetes, or suffering from other relevant health conditions; or c) were not performed in human subjects. Studies that did not analyze microbiota composition were not included. Figure 1 depicts how the selection of articles was made. The initial search (which included the following filters: Child: 6-12 years old and adolescent 13-18 years old) resulted in 409 references, of which 379 were excluded after reading the title due to participants having other pathologies but not OW or OB (Fig. 2.) Thirty full articles were chosen for further screening. Two articles were excluded because they did not provide any information on the number of participants. Later, 12 out of 28 articles were excluded because they did not include any data on microbiota composition. Finally, 16 articles were selected for this systematic review: 12 observational studies and 4 intervention studies (3 randomized and 1 non-randomized)..
To classify children or adolescents into OW or OB categories, studies used the following indicators: BMI (22–26) BMI-z score (27–32), or BMI percentile (33–35). For the gut microbiota sample analysis, all studies (22–33,35–38) used fecal samples and gut microbiota was analyzed by 16S rRNA sequencing.
3. RESULTS AND DISCUSSION
Sixteen articles were selected for this systematic review: 12 observational studies and 4 intervention studies (3 randomized and 1 non-randomized).
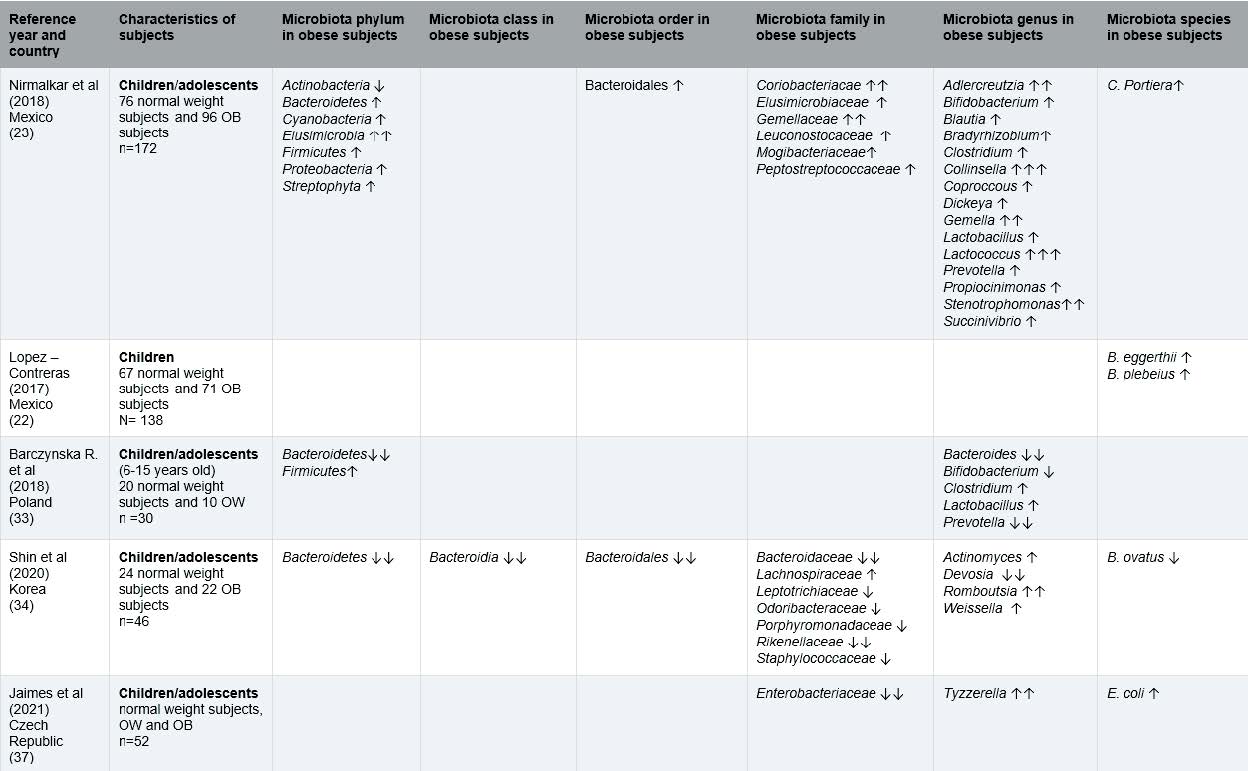
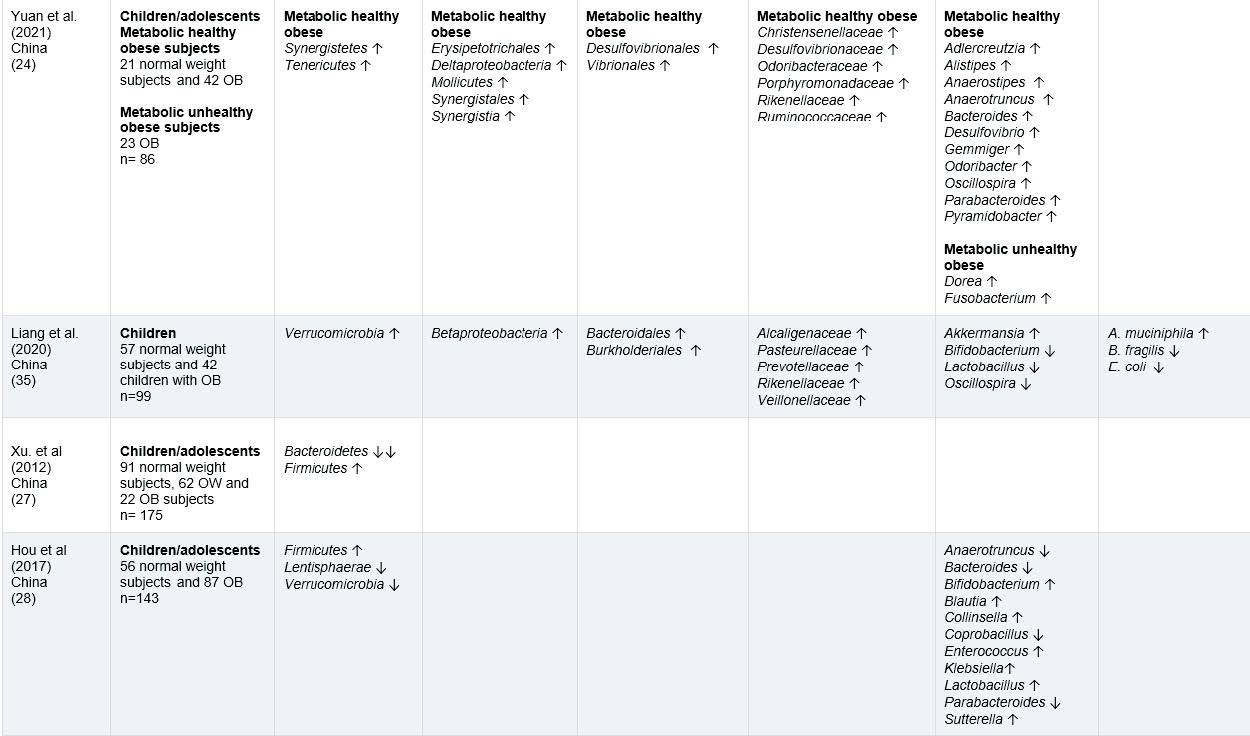
The information on gut microbiota of children and adolescents with OW or OB from 12 observational studies is included in tables 2, while tables 4 highlights the interesting findings. A total of 1,481 participants, male and females, from the following countries: Spain (26), Brazil (25) Mexico (22,23), Poland (33), Korea (34), Czech Republic (37), China (24,27,28,35) and Netherlands (29).
Regarding intervention studies, Tables 3 provide a description of the changes in gut microbiota composition after interventions. In addition, Table 5, presents the most interesting findings from these studies. They included 161 participants, males, and females, from the following countries: Spain (38), Mexico (30), Canada (31), and South Korea (32). The intervention duration ranged from 6 to 16 weeks with an average of 10 and a half weeks. Four types of intervention related to lifestyle modifications were identified in relation to diet and physical activity.
Table 1: Criteria for including articles in this systematic review
Figure 1. Flow diagram of the article selection process for this systematic review
The 1st study ran for 16 weeks in pediatric subjects with OW and OB. Participants were given an oligofructose-enriched inulin (OI; 8 g/day) or maltodextrin (a placebo with an isocaloric dose) which was the control group once a day (38).
The 2nd study consisted of a 12-week combined strength and endurance training program with 2 sessions /week in OB subjects compared to a control group (normal weight) (30).
The 3rd study ran for 6 weeks, combining physical activity (150 min/ week) and a dietary intervention (increasing fruit and vegetable consumption, replacing refined carbohydrates with whole grains, and limiting the consumption of sugar added to processed food and sugar-sweetened beverages) in OB subjects (31).
The 4th study was an 8-week weight reduction program with 3 visits receiving counseling from dietitians, personal trainers, research nurses, and pediatric clinicians, in which OB participants were allocated in either a fat loss group or fat gain group, compared to normal- weight children (32).
The results from the observational and intervention studies (Table 2 and Table 3) will be discussed in terms of taxonomic classifications separated by phylum, order, family, genus, and species. In the results, we will discuss the most significant findings -those that had similar outcomes at least in 2 independent studies-, which are presented in Table 4 and Table 5.
3.1. Observational Studies
First, we will comment on the baseline results combining observational and intervention studies regarding the phylum level analysis of gut microbiota. A higher abundance of Firmicutes in children/adolescents with OB compared to normal weight in six studies was found, from which 5 were observational (23,26–28,33) and one was an intervention study (31). Firmicutes levels in adults have also been described to be increased (39) and related to increased consumption of food rich in animal fat in children (33). This bacterium is positively correlated with serum tumor necrosis factor alpha (TNF-alfa) which is closely related to inflammatory levels. (28).
Notably, four studies; three observational (27,33,34), and one intervention study (32), reported a lower abundance of Bacteroidetes in children/adolescents with OW/OB when compared to normal weight. Bacteroidetes increase energy absorption (27) and a negative relationship between their dominance and body weight is found in adults with OW/OB (33). Results in this review of low levels of Bacteroidetes and high levels of Firmicutes are supported by previous adult studies, which indicate a higher Firmicutes: Bacteroidetes ratio related to OB and inflammation levels (34,40–42).
Four studies; two observational (23,26) and two intervention studies (30,31) indicated a higher abundance of Proteobacteria in children/adolescents with OW/OB when compared to normal weight (23,30,31) and metabolically unhealthy obese (MUO) compared to metabolically healthy obese (MHO) (26). In adult studies low abundance of this phylum have been associated with a healthy intestinal microbiota (39). Recent studies have focused on the identification of Proteobacteria, which might be implicated in the genesis of endotoxemia and in the development of metabolic disorders (44). It can be concluded that Proteobacteria can be a potentially pathogenic species that induces potentially high inflammatory burdens (45).
Two observational studies; one in children/adolescents with OW/OB when compared to normal weight (28) and the other in metabolically unhealthy obese vs. metabolically healthy obese children/adolescents (26), found Verrucomicrobia in lower abundances. Some research works suggest that Verrucomicrobia could play an important role in maintaining a healthy gut microbiota, as it is found to be in less abundance in gut microbiota of adults with OB, and that weight loss resulted in an increase in Verrucomicrobia abundance (46)
Regarding the order-level analysis of gut microbiota, the relevant findings were that three observational studies found a higher abundance of Bacteroidales in children/adolescents with OB when compared to normal weight (23,35) and in metabolically unhealthy obese vs. metabolically healthy obese (26). One study also found the same results in adults with OB (47).
At genus’s level analysis of gut microbiota, two studies found a lower abundance of Bifidobacterium in children/adolescents with OW/OB compared to those with normal weight subjects (33,35). Similarly, Bifidobacterium is found in lower concentrations in adults with OB (28,31,38,48). Bifidobacterium is considered a common beneficial bacterium in the human body (31,35,48) and has been linked to inhibiting cholesterol absorption and reducing the risk of OW/OB in childhood (31). Additionally, Bifidobacterium has been shown to improve digestive health by reducing constipation (49),and to stimulate the immune system, thereby helping protect against infections and diseases (50). Moreover, Bifidobacterium may reduce obesity-associated inflammation by restoring the lymphocyte macrophage balance and reducing the abundance of Firmicutes (28)
Three studies showed a higher relative abundance of Blautia; with two observational studies in children/adolescents with OW/OB compared to normal weight (23,28), and one intervention study in children/adolescents with OB compared to normal weight (32). In adult studies, the abundance of Blautia was significantly higher in individuals with OB compared to normal weight in adults, and positively associated with OB and metabolic syndrome (51).
Two studies described a higher abundance of Clostridium in children/adolescents with OW/OB compared to normal weight (23,33). Higher Clostridium levels are found in children with weight gain (33). In adult studies Clostridium has also been found to be higher abundance in OB subjects (39), involved in regulating energy metabolism and influencing weight gain (52). Clostridium has been related to eating food rich in animal fat (33) which has a potential to induce weight gain, systemic inflammation, and metabolic alterations (53).
Lactobacillus was described to have higher abundance in three studies with children/adolescents with OW/OB compared to normal weight (23,28,33). Higher levels of Lactobacillus have been found in OB adults than in normal weight (48). Some species belonging to this genus have been indeed associated with OB whereas others have been found to be associated with weight loss in humans (48).
Akkermansia was reported in lower abundance in two studies of children/adolescents with OW/OB compared to normal weight (29) and in metabolically unhealthy obese vs. metabolically healthy obese children/adolescents (26). Akkermansia was found in higher abundances in OB adults (39) and associated with leanness in both adult and children’s studies (26). A lower abundance is associated with a higher risk of metabolic disease and developing one or more cardiovascular diseases (26).
In the species level, two studies reported a higher abundance of Escherichia coli in children/adolescents with OW/OB compared to normal weight (26,37). Some studies suggest this species may be associated with OB in adults as it has been found to increase body weight and adiposity and induce impaired glucose intolerance (54). Additionally, research has suggested that Escherichia coli may contribute to maternal obesity during the perinatal period by exacerbating inflammation- induced gut epithelial barrier leak, which can in turn lead to metabolic dysregulation (55). The membrane of Escherichia coli contains a lipopolysaccharide (LPS), which is found in gram negative bacteria (56). High levels of LPS in the bloodstream and intestine can trigger systemic inflammation (56), and can directly and indirectly contribute to the inflammatory reaction in adipose tissue during OB (57).
3.2. Intervention Studies
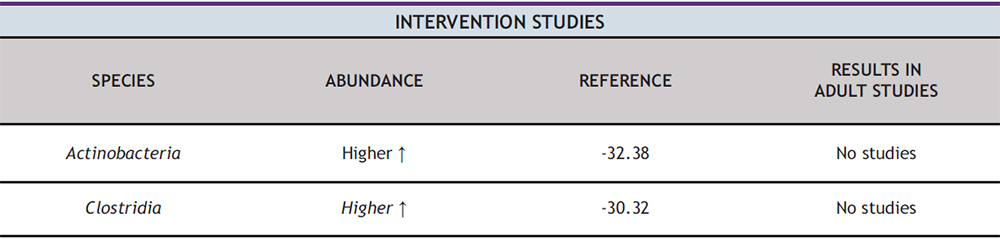
At the phylum-level analysis, two studies found a higher proportion of Actinobacteria after intervention. In study 1°, which used supplementation with oligofructose enriched inulin compared to a placebo group, a higher abundance of Actinobacteria was observed after the intervention (38). Similarly, study 4 °, which implemented a weight reduction program in children/adolescents with OB compared to normal weight subjects, also reported a higher proportion of Actinobacteria (32). Certain species of Actinobacteria such as Bifidobacterium, have been found to have potential health benefits in adults. Bifidobacterium can help improve gut health, enhance immune function and protect against infection. Additionally, it can break down fiber, providing energy and promoting intestinal health (58).
Table 2: Summary of observational studies on microbiota composition in children/adolescents with OW/OB. Abbreviations MHO, MUH, OB and OW.
Table 3: Summary of intervention studies regarding changes in microbiota composition after lifestyle interventions in children/adolescents with OW/OB
At the class-level analysis, a higher abundance of Clostridia was found in two studies after intervention. In the 2° study, which consisted of a combined strength and endurance training program in children with OB compared to normal weight subjects, a higher abundance of Clostridia was observed (30). Similarly, study 4°, which implemented a weight reduction program in children/adolescents with OB compared to normal weight, also reported a higher proportion of Clostridia (32).
Table 4: Summary of the principal results found in observational and baseline intervention studies on the composition of gut microbiota in children/adolescents with OW or OB
Table 5: Summary of the principal results found after interventions on the composition of gut microbiota in children/adolescents with OW or OB
Our article has limitations and strengths. One limitation is that we excluded subjects diagnosed with diseases such as autism, diabetes, or suffering from other relevant health conditions. Another limitation is that there were 16 articles that fulfill the inclusion criteria.
Notably, the strength of our work is based on the study design, a systematic review that compiles the evidence on microbiota composition in children with OW or OB. Out of 409 articles,16 articles were selected for this systematic review. With regard to the results, it is worth mentioning that some of them are supported by previous findings in adult populations with obesity.
4. CONCLUSION
This systematic review compiled the evidence on the structural characteristics of gut microbiota in children and adolescents with OB or metabolically unhealthy obesity compared to those with normal weight, through observational and intervention studies. It’s known that OB can disrupt the frequent distribution of microorganisms in the gastrointestinal tract, resulting in an imbalance in the ecological composition of the gut microbiota, decreasing microbiota diversity and increasing the number of species associated with inflammation. The results are consistent with previous findings in adult populations, highlighting the impact of OB on gut microbiota. However, there is a limited amount of research on this topic in children and adolescents (36,43), and studies with larger sample sizes are needed to understand the composition of gut microbiota. This information will be useful for designing dietary interventions to prevent or reverse dysbiosis in individuals with OB.
5. REFERENCES
- Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017; 16: 1823-36.
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14.
- Frye RE, Lizcano F, Martini S, Indrio F, Francavilla R, Corvaglia L, et al. Epigenetic Matters: The link between early nutrition, microbiome, and long-term health development. Front Pediatr. 2017; 5:178.
- 3º Curso sobre obesidad y síndrome metabólico. An Real Acad Farm 2016; 82 (2).
- Wilson M. The human microbiota in health and disease: an ecological and community-based approach. 2018. Garland Science.
- López Goñi I. Microbiota. Los microbios de tu organismo. Guadalmazán. 2018;20-43.
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014; 505(7484):559–63.
- Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology. 2017; 152(7):1671–8.
- Stephens RW, Arhire L, Covasa M. Gut microbiota: from microorganisms to metabolic organ influencing obesity. Obesity. 2018; 26(5):801–9.
- De Cedrón MG, De Molina AR. Precision nutrition to target lipid metabolism alterations in cancer. Precision Medicine for Investigators, Practitioners and Providers / Faintuch J, Faintuch S, ed. 2020, ISBN 978-0-12-819178-1, pg. 291-299.
- Wang W, Yan Y, Yu F, Zhang W, Su S. Role of oral and gut microbiota in childhood obesity. Folia Microbiol (Praha).2023; 68:197-206.
- Shi-Yu C, Cai-Ning Z, Xiao-Yu X, Guo-Yi T, Harold C, Ren-You G, Hua-Bin L, et al. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci Technol. 2019; 92: 194-204.
- Socol CT, Chira A, Martinez-Sanchez MA, Nuñez-Sanchez MA, Maerescu CM, Mierlita D, et al. Leptin signaling in obesity and colorectal cancer. Int J Mol Sci. 2022; 23(9):4713.
- Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851.
- WHO. Obesity and overweight. [accessed 14 Mar 2023] Available from: https://www.who.int/news-room/fact- sheets/detail/obesity-and-overweight
- BMI-for-age (5-19 years). Available from: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age [accessed 14 Mar 2023]
- Obesity. Available from: https://www.who.int/health-topics/obesity#tab=tab_1 [accessed 15 Mar 2023]
- Europe: one in three children overweight or obese. Available from: https://unric.org/en/europe-one-in-three-children-overweight-or-obese/ [accessed 15 Mar 2023]
- Brief report surveillance study on nutrition, physical activity, child development and obesity. Estudio Aladino 2019. Madrid, september 2020. Available from: https://www.aesan.gob.es/AECOSAN/docs/documentos/nutricion/observatorio/Brief_report_ALA DINO_2019_NAOS.pdf [accessed 14 Feb 2024].
- WHO European Childhood Obesity Surveillance Initiative (COSI) Report on the fourth round of data collection. 2021. Available from: http://apps.who.int/bookorders. [accessed 14 Feb 2024].
- Noncommunicable diseases: Childhood overweight and obesity. Available from: https://www.who.int/news-room/questions-and-answers/item/noncommunicable-diseases-childhood-overweight-and-obesity [accessed 14 Mar 2023]
- López-Contreras BE, Morán-Ramos S, Villarruel-Vázquez R, Macías-Kauffer L, Villamil-Ramírez H, León-Mimila P, et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr Obes. 2018;13(6):381–8.
- Nirmalkar K, Murugesan S, Pizano-Zárate ML, Villalobos-Flores LE, García-González C, Morales-Hernández RM, et al. Gut microbiota and endothelial dysfunction markers in obese mexican children and adolescents. Nutrients. 2018;10(12).
- Yuan X, Chen R, Mccormick KL, Zhang Y, Lin X, Yang X. The role of the gut microbiota on the metabolic status of obese children. Microb Cell Fact. 2021; 20(1):53
- Ignacio A, Fernandes MR, Rodrigues VAA, Groppo FC, Cardoso AL, Avila-Campos MJ, et al. Correlation between body mass index and faecal microbiota from children. Clin Microbiol Infect. 2016; 22(3):258.e1-258.e8.
- Alcazar M, Escribano J, Ferré N, Closa-Monasterolo R, Selma-Royo M, Feliu A, et al. Gut microbiota is associated with metabolic health in children with obesity. Clin Nutr. 2022; 41(8):1680–8.
- Xu P, Li M, Zhang J, Zhang T. Correlation of intestinal microbiota with overweight and obesity in Kazakh school children. BMC Microbiol. 2012;12.
- Hou YP, He QQ, Ouyang HM, Peng HS, Wang Q, Li J, et al. Human gut microbiota associated with obesity in Chinese children and adolescents. Biomed Res Int. 2017: 2017: 27585989
- Mbakwa CA, Hermes GDA, Penders J, Savelkoul PHM, Thijs C, Dagnelie PC, et al. Gut microbiota and body weight in school-aged children: The KOALA Birth Cohort Study. Obesity (Silver Spring). 2018; 26(11):1767–76.
- Quiroga R, Nistal E, Estébanez B, Porras D, Juárez-Fernández M, Martínez-Flórez S, et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med. 2020; 52(7):1048–61.
- Morán-Ramos S, Siliceo-Bernardi MT, Villalpando-Carrión S, Canizales-Quinteros S, Frigolet ME, Gutiérrez-Aguilar R. Gut microbiota composition after a dietary and physical activity intervention: a pilot study in Mexican children with obesity. Bol Med Hosp Infant Mex. 2022;79(5):318–25.
- Cho KY. Lifestyle modifications result in alterations in the gut microbiota in obese children. BMC Microbiol. 2021; 21(1).
- Barczyńska R, Litwin M, Sliżewska K, Szalecki M, Berdowska A, Bandurska K, et al. Bacterial Microbiota and fatty acids in the faeces of overweight and obese children. Pol J Microbiol. 2018;67(3):339–45.
- Shin S, Cho KY. Altered gut microbiota and shift in bacteroidetes between young obese and normal-weight Korean children: a cross-sectional observational study. Biomed Res Int. 2020;2020: 6587136.
- Liang C, Guo M, Liu T, Zhou X, Gong P, Lyu L, et al. Profiles of gut microbiota in children with obesity from Harbin, China and screening of strains with anti-obesity ability in vitro and in vivo. J Appl Microbiol. 2020; 129(3):728–37.
- Santacruz A, Marcos A, Wärnberg J, Marti A, Martin-Matillas M, Campoy C, et al. EVASYON Study Group. Obesity (Silver Spring). 2009; 17:1906-15.
- Jaimes JD, Slavíčkova A, Hurych J, Cinek O, Nichols B, Vodolanova L, et al. Stool metabolome-microbiota evaluation among children and adolescents with obesity, overweight, and normal-weight using 1H NMR and 16S rRNA gene profiling. PLoS One. 2021; 16(3).
- Nicolucci AC, Hume MP, Martínez I, Mayengbam S, Walter J, Reimer RA. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017; 153(3):711–22.
- Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes. 2018; 9(4):308–25.
- Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2015; 2(8):968.
- Álvarez-Mercado AI, Navarro-Oliveros M, Robles-Sánchez C, Plaza-Díaz J, Sáez-Lara MJ, Muñoz-Quezada S, et al. Microbial population changes and their relationship with human health and disease. Microorganisms. 2019; 7(3).
- Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017; 19(1):95–105.
- Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri JM, Moreno LA, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond). 2009; 33:758-67.
- Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: a common factor in human diseases. Biomed Res Int. 2017; 1–7.
- Zhang S, Dang Y. Roles of gut microbiota and metabolites in overweight and obesity of children. Front Endocrinol (Lausanne). 2022; 13:2173.
- Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–5.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006; 444(7122):1027–31.
- Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016; 73(1):147–62.
- Tabbers MM, de Milliano I, Roseboom MG, Benninga MA. Is Bifidobacterium breve effective in the treatment of childhood constipation? Results from a pilot study. Nutr J. 2011;10(1):19.
- Plaza-Díaz J, Ruiz-Ojeda F, Vilchez-Padial L, Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017; 9(6):555.
- Geng J, Ni Q, Sun W, Li L, Feng X. The links between gut microbiota and obesity and obesity related diseases. Biomed Pharmacother. 2022; 147:112678.
- Million M, Lagier J-C, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infect. 2013; 19(4):305–13.
- Jamar G, Santamarina AB, Dias GC, Masquio DCL, de Rosso VV, Pisani LP. Relationship between fatty acids intake and Clostridium coccoides in obese individuals with metabolic syndrome. Food Res Int. 2018; 113:86–92.
- Ju T, Bourrie BCT, Forgie AJ, Pepin DM, Tollenaar S, Sergi CM, et al. The gut commensal Escherichia coli aggravates high-fat-Diet-induced obesity and insulin resistance in mice. Appl Environ Microbiol. 2023; 89(3).
- Dreisbach C, Morgan H, Cochran C, Gyamfi A, Henderson WA, Prescott S. Metabolic and microbial changes associated with diet and obesity during pregnancy: what can we learn from animal studies? Front Cell Infect Microbiol. 2022; 11:795924.
- Schultz KM, Klug CS. Characterization of and lipopolysaccharide binding to the E. coli LptC protein dimer. Protein Sci. 2018; (2):381–9.
- Hersoug L-G, Møller P, Loft S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr Res Rev. 2018; 31(2):153–63.
- Turroni F, Ventura M, Buttó LF, Duranti S, O’Toole PW, Motherway MO, et al. Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cell Mol Life Sci. 2014; 71:183–203.