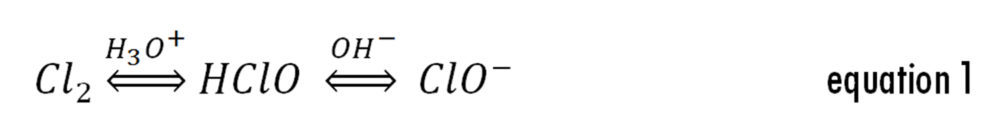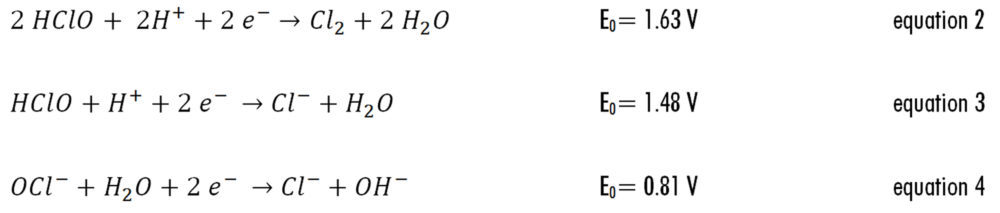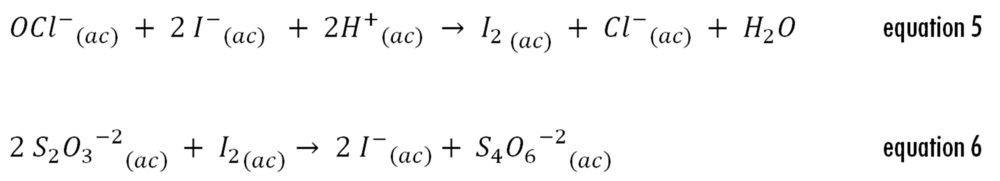RESUMEN:
Introducción y Objetivos: En el contexto de COVID-19, la Organización Mundial de la Salud recomienda el uso de soluciones de hipoclorito de sodio 1 g/L, como una concentración capaz de inactivar el SARS-CoV-2 y la gran mayoría de patógenos presentes en el entorno sanitario. En consecuencia, hay un renovado interés en realizar estudios de estabilidad de estas disoluciones. El objetivo de este trabajo es verificar la concentración de cloro activo en varias marcas comerciales de hipoclorito de sodio y proponer una fecha límite de uso para soluciones de 1 g/L, obtenidas por dilución con agua potable proveniente de diferentes fuentes.
Métodos: La concentración de cloro activo de preparaciones comerciales con concentración nominal entre 25-60 g/L fue valorada por titulación iodométrica. A partir de una de las marcas comerciales se prepararon diluciones de 1 g/L usando agua proveniente de diferentes plantas potabilizadoras en Córdoba, Argentina. Las disoluciones se almacenaron a temperatura ambiente, tanto expuestas como protegidas de la luz y fueron posteriormente tituladas a intervalos de tiempo preestablecidos. La fecha límite de uso se alcanzó cuando la concentración de cloro activo cayó por debajo del 90 % de la inicial.
Resultados: La concentración de cloro activo en las soluciones comerciales estuvo dentro de los valores establecidos por la normativa vigente. Las diluciones protegidas de la luz mostraron una disminución menor al 10 % en la concentración de cloro activo durante los primeros 10 días de ensayo. Sin embargo, una muestra superó el límite de aceptación luego de 14 días. En contraste, en las muestras expuestas a la luz, la concentración de cloro activo cayó a 96.4 % a las 24 horas y 79.3 % después de 48 horas. No se observaron diferencias relacionadas con las fuentes de agua potable.
Conclusiones: se confirmó la concentración nominal de cloro activo en todas las marcas comerciales evaluadas. Independientemente de la fuente de agua potable utilizada para la dilución, las soluciones de 1 g/L fueron estables durante 10 días cuando se almacenaron a temperatura ambiente y protegidas de la luz. En contraste, las soluciones expuestas a la luz mantienen la concentración de cloro activo durante solo 24 horas.
Palabras Clave: coronavirus, desinfección, blanqueadores, control de infecciones, control de calidad
ABSTRACT:
Introduction and objectives: In the context of COVID-19, the World Health Organization has recommended the use of extemporaneously prepared bleach solutions of 1 g/L, as a conservative concentration able to inactivate SARS-CoV-2 and the vast majority of other pathogens that may be present in the healthcare setting. Consequently, there is a renewed interest in conducting stability studies of these solutions. The goal of this work was to verify the available chlorine concentration in several bleach solutions trademarks and to propose a beyond use date for 1 g/L bleach solutions, obtained after dilution with drinking water from different sources.
Methods: Bleach trademarks, with nominal concentrations between 25-60 g/L, were subjected to iodometric titration to determine the available chlorine concentration. One trademark was used to prepare 1 g/L dilutions using water from different purification plants in Córdoba, Argentina. The samples were stored at room-temperature, both exposed or protected from light. The available chlorine concentration was determined by titration at preestablished time intervals. The beyond use date was reached when the available chlorine concentration dropped below 90 % of its initial.
Results: The concentration of active chlorine in the different trademark bleaches was within the values established by current regulations. Diluted solutions protected from light showed a decrease of less than 10 % in active chlorine concentration during the first 10 days of assay. However, one sample exceeded the acceptance limit after 14 days. In contrast, in the samples exposed to light, the concentration of active chlorine dropped to 96.4 % at 24 hours and 79.3 % after 48 hours. No differences related to drinking water sources were observed.
Conclusions: Compliance of the nominal available chlorine concentration in trademark bleach solutions was confirmed. Regardless the water source used for dilution, 1 g/L bleach solutions were stable for 10 days when stored at room temperature and protected from light. Instead, solutions exposed to light maintain their available chlorine concentration for only 24 hours.
Keywords: coronavirus, disinfection, bleaching agents , infection control, quality control
1. INTRODUCCIÓN
The recent emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing COVID-19 is a major burden for health care systems worldwide. Consistent and correct disinfection procedures are of enormous importance.
Bleach, also known as sodium hypochlorite solution, is a germicidal agent, commonly used for surface disinfection. It is fast-acting and effective against bacteria, fungi and viruses, even when used in very low concentrations. It is also widely available at a low cost (1–4).
Sodium hypochlorite solution is a strong oxidizing agent, in which the CI atom of the available chlorine species, i. e. hypochlorous acid (HOCl) and hypochlorite ion (OCl–) behave as Cl+, a strong electrophile that reacts with substances of high electronic densities (5).
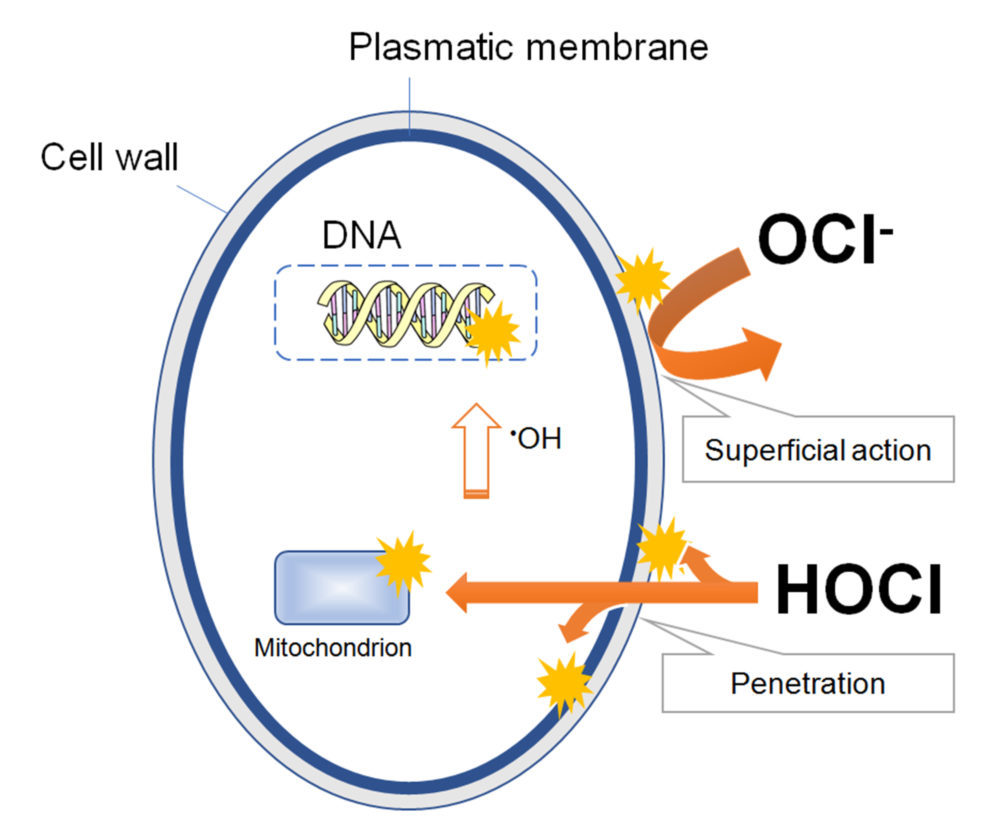
The germicidal activity of sodium hypochlorite solutions mainly depends on the concentration of HOCI species, since it can passively diffuse through the cell wall and plasmatic membrane of microbes, affecting both the exterior and interior of the cell. On the contrary, the ionized OCl– has a low germicidal activity due to its limited ability to diffuse through the microbial cell wall, and mainly acts oxidizing the cell surface (Figure 1).
Figure 1. A model that represent the mechanisms of the germicidal actions of HOCl and OCl– based on their ability to penetrate the membrane into the microbial cell. Adapted from reference (5).
The relative concentration of available chlorine species is significantly affected by the solution pH, which is known to also affect the chemical stability of the solution (5).
As shown in equation 1, Cl2 predominates at acid pH; however, it easily escapes from the solution since it is a low water soluble gas. As the solution pH increases, the equilibrium shifts towards the formation of the HOCl species (prevalent at pHs between 5.5-9.5) and OCl- (pHs above 11).
As can be seen in equations 2 to 5, the standard electrode potentials for the reduction of HOCI is higher than that of OCl– (5), which means that HOCl species is more reactive; the challenge for practical use of bleach is that although chlorine solution shelf-life is greatest at pH>11 (pH of concentrated solutions), it is more effective in disinfecting at pH <8 (pH of diluted solutions) (6,7).
Concentrated trademark presentations of bleach with available chlorine concentrations between 20 to 110 g/L are regulated as household products.
In the context of COVID-19, the World Health Organization (WHO) has recommended the use of extemporaneously prepared bleach solutions of 1 g/L, as a conservative concentration able to inactivate SARS-CoV-2 and the vast majority of other pathogens that may be present in the healthcare setting (8). To ensure the proper disinfection, concentration and time of application are of great concern.
Some authors have determined that bleach solutions of 0.5 g/L and 5 g/L remain stable for several days (6,9), however, the chemical stability of 1 g/L solutions has not yet been demonstrated.
It is widely known that concentrated bleach solutions have an expected and permitted reduction in the available chlorine concentration from up to 33 % during the 150 days of shelf-life (10), which may affect significantly the concentration of diluted solutions if they are made based on the nominal labeled concentration. In addition, the effect of light, heat, contact with air, and the presence of traces of metals, metal ions and organic matter can accelerate chemical degradation (5,7,11,12, 13).
In Cordoba City (Argentina), drinking water comes from different sources as lakes, damps and waterholes, and is processed in different purification plants. Although the quality of drinking water can be guaranteed, there is a wide range of acceptable limits in their mineral and organic content, which could affect the chemical stability of diluted bleach.
Finally, the unusually high demand for bleach (and other sanitary products) during COVID-19, may exceed the control capacity of sanitary authorities, generating a favourable field for the appearance of counterfeit or adulterated products (14–16), which suggests the convenience of performing some quality controls.
The objectives of this work were to determine the compliance of the available chlorine concentration in trademark bleach solutions and to propose a beyond use date (BUD) for 1 g/L bleach solutions obtained using different drinking water sources as diluent.
2. Materials and methods
2.1 Water sources
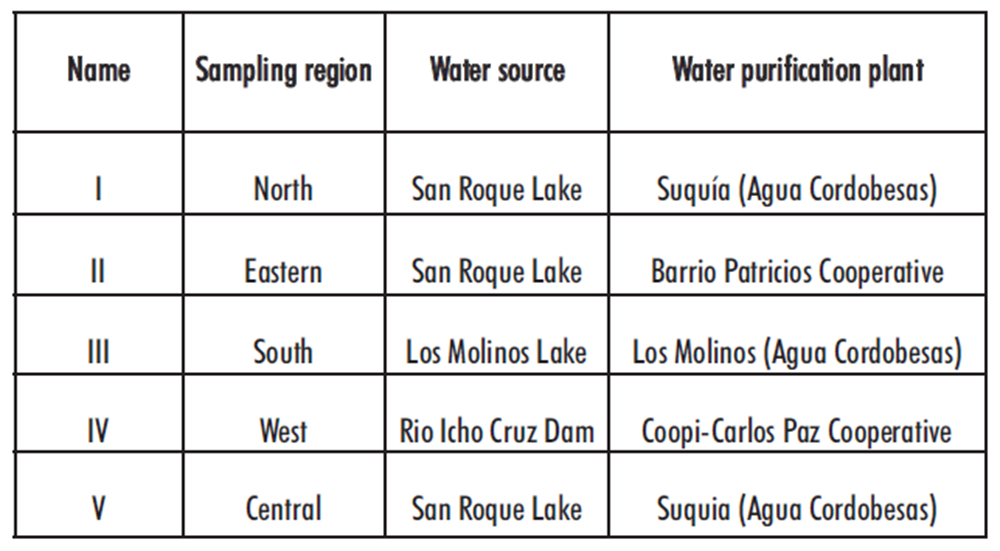
Samples of drinking water were taken from five different sources around Córdoba city, named from I to V (see table 1). To avoid variations related to water storage conditions, samples were taken directly from the drinking water network, from the tap located at the previous entrance to the tank. Their conductivity and pH were directly measured with a Mettler Toledo pHmeter, at 250C using a 2-steel poles conductivity sensor and a glass electrode of Ag/AgCl, respectively. The Chemical Oxygen Demand (COD) was determined by the Standard Methods for the examination of water and wastewater (5220 D) with a detection/quantification limit of 15/42 mg/L of O2 (17) in the Center of Applied Chemistry (CEQUIMAP; Cordoba, Argentina). Values of pH between 6.5-8.5, conductivity below 1000 µS/cm were considered acceptable. The COD levels are not established for drinking water, however, it is known that surface waters present values of 20 mg/L of O2 or less in unpolluted waters up to more than 200 mg/L of O2 in waters receiving effluents (18).
3. Bleach commercial solutions
Three trademark bleach presentations, with expiration dates within the next 150 days and nominal concentration between 25 to 60 g/L, were used in this study. The new containers were opened on the day of the assay.
4. Available chlorine titration
Reagents: Potasium iodide (analytical grade; Pura Química ®, Argentina), glacial acetic acid 99.5 % (pro-analysis quality, Cicarelli ®, Argentina), sodium thiosulfate 0.05 M (normalized analytical reagent Anedra ®, Argentina) and distilled water (Regondi ®, Argentina). Starch solution was prepared dissolving 1 g of soluble starch in 200 mL of water as described in Farmacopea Argentina 7 (FA 7) (19).
Procedure: Immediately after reception the concentrated bleach solutions were titrated as described by FA 7 (19). Briefly, 1 mL of the concentrated bleach solutions was diluted 1:50 with distilled water. An exactly measured 1 mL aliquot of this dilution was added to a glass flask containing 5 mL of distilled water and 0.5 mL of glacial acetic acid. Then, 0.10 g of potassium iodide were added to react with the available chlorine species producing a stoichiometric amount of molecular iodine (equation 5) to be titrated with 0.05 M sodium thiosulfate (equation 6). Just before reaching the theoretical end point, a few drops of starch solution were added. Each mL of 0.05 M sodium thiosulfate solution consumed in the reaction was equivalent to 3.723 mg of sodium hypochlorite. Additionally, a blank titration was performed and the volume of sodium thiosulfate consumed was discounted from the volume of the end point. The titrations were performed in triplicate. The pH of the solutions before and after titration was 2.40 ± 0.05.
5. BUD determination for diluted beach solution
The 1 g/L solutions were prepared by diluting concentrated bleach with drinking water, based on the labeled concentration of available chlorine. Briefly, 2.2 mL of trademark A (46 g/L of available chlorine) were added to a 100 mL volumetric flask and taken to final volume with drinking water coming from five different sources (I to V, see table 1).
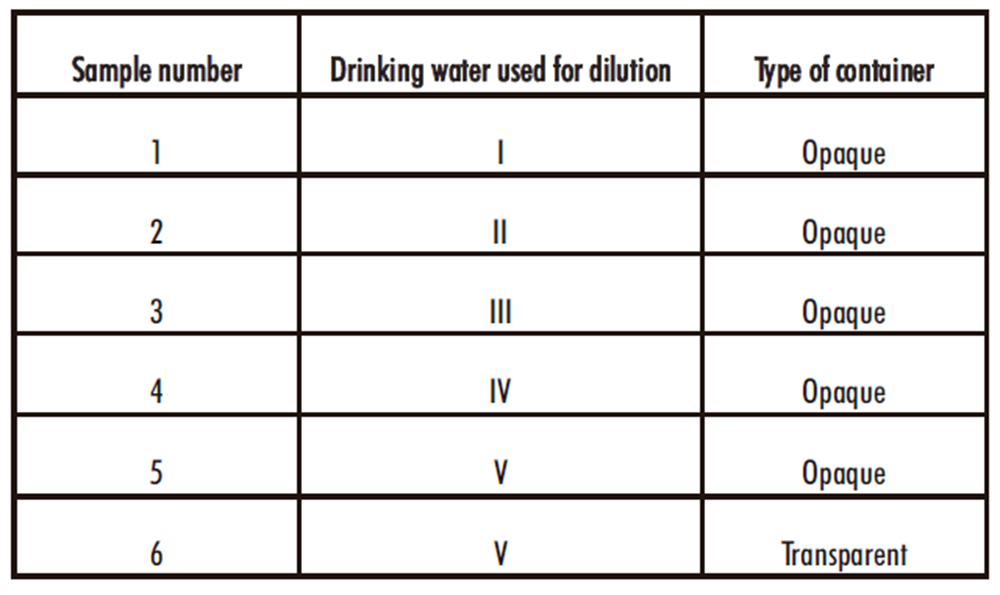
Table 1. Sources of drinking water used to prepare bleach dilutions in this study.
The diluted solutions were transferred to disinfected plastic bottles, tightly closed and stored at room-controlled temperature (20-25 ºC) under the conditions described in table 2. In addition, a solution obtained with drinking water number V was transferred to a transparent container and exposed to room light.
Table 2. Conditions of preparing and storage of diluted bleach solutions
To determine chlorine concentrations, 1 mL of each 1 g/L solution was titrated (in triplicate) at 0, 1, 12 hours and 1, 2, 3, 5, 7, 10 and 14 days, using the experimental procedure described for the concentrated bleach solutions. The BUD of the diluted solutions were considered achieved when the average chlorine dropped below 90 % of its initial concentration. The normal chlorine amount contained in the different drinking water sources was considered negligible.
6. Results and discussion
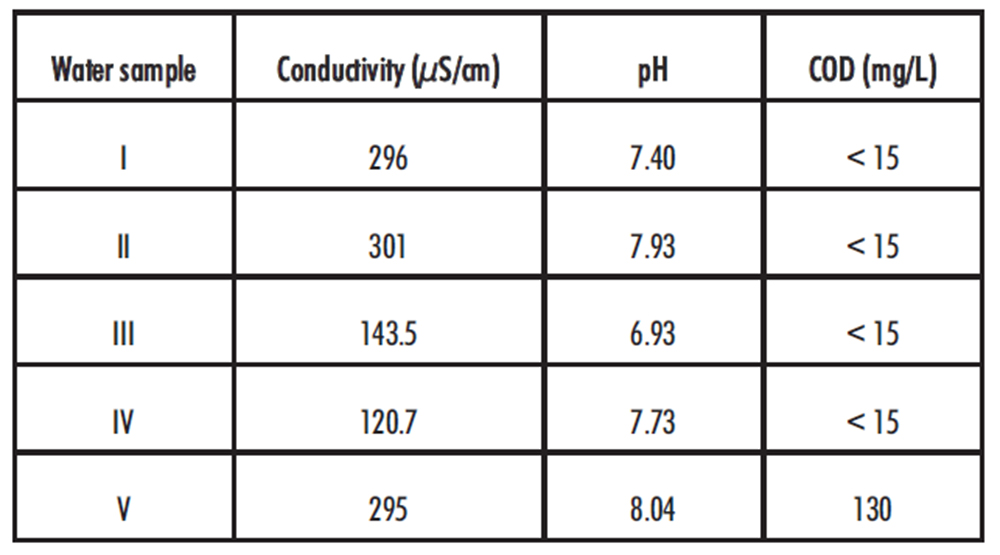
The pH, conductivity and COD values of drinking water samples were within the limits established by international recommendations (18). However, sample Nº 5 has a COD value higher than expected (table 3).
Table 3. Drinking water analysis
The WHO has proposed the use of 1 g/L bleach solutions for disinfection in institutional settings since it provides a large margin of safety regarding the effective concentration necessary to inactivate the majority of microorganisms on inert surfaces.
Bleach solutions are usually prepared based on standard procedures that consider the labeled available chlorine of the concentrated bleach, which is known to decrease with time. Thus, the confirmation that nominal concentration is maintained within the accepted range during the shelf-life period is an additional warranty that the expected concentrations can be obtained after dilution, provided that correct procedures are followed.
Table 4 shows the available chlorine of the assayed concentrated solutions. As can be seen there, the values obtained are in agreement with those declared in their respective labels and also with the concentration decrease permitted for the time elapsed from the date of elaboration (Argentinian regulation 7355/2019) (10).
Table 4. Available chlorine concentration in several trademark bleaches as determined by iodometric titration.
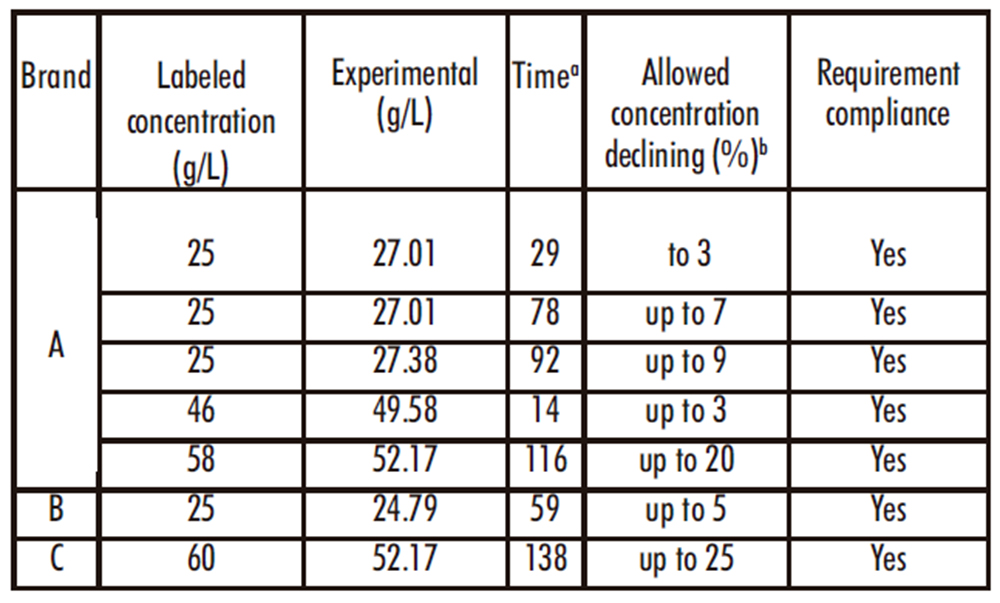
a Time elapsed from the manufacturing date and the day of titration
b Allowed reduction of available chlorine concentration considering the time elapsed from the date of elaboration (Argentinian regulation 7355/2019).
Notice that the standard iodometric titration requires acidification of the solution in order to shift the equilibrium towards the formation of HClO as the only species. Therefore, the results obtained correspond to the total available chlorine.
Since bleach dilutions are usually performed with drinking water, which is known to contain salts, metals and organic matter, it is important to know if the different water sources of the city have an impact on the solutions’ BUDs.
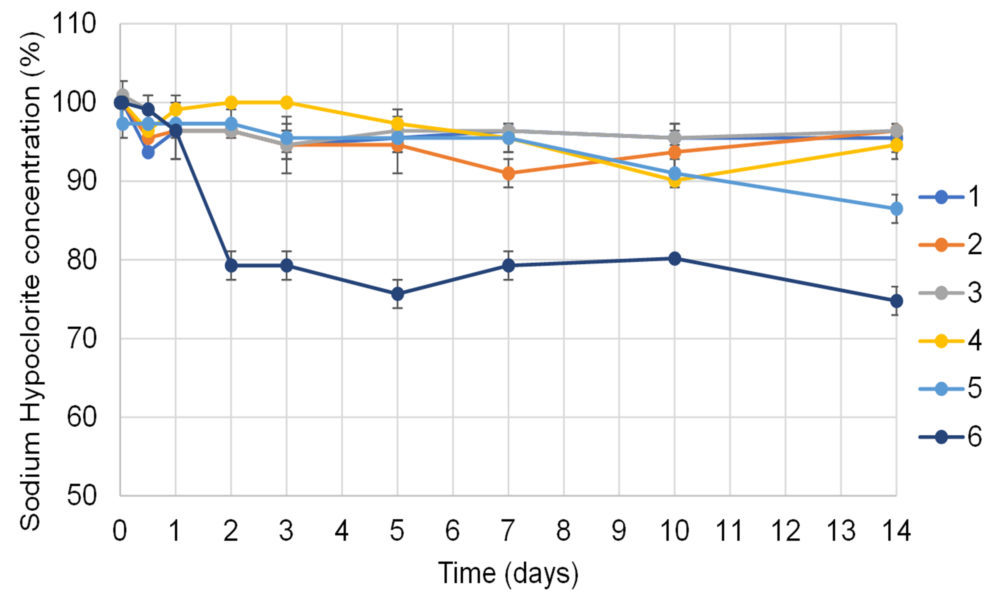
Figure 2 shows the variation of available chlorine in 1 g/L bleach solutions relative to their initial concentration.
Figure 2. Mean available chlorine concentrations determined in 1 g/L bleach solutions studied over a 14-days period. All the samples were prepared using drinking water from different sources around Cordoba City as diluent. All samples were stored at room-controlled temperature in opaque containers, except for sample number 6, which was stored in a transparent container and exposed to light.
During the first 10 days of assay all the solutions protected from light showed less than 10% decrease in the available chlorine concentrations. On the 14th day, however, the concentration of available chlorine in sample N° 5 fell below the acceptance limit for the BUD (13.5%). This behaviour can be related to the higher COD value observed in its respective water sample. In addition, the differences in water pH and conductivity could also explain the small variations observed among the solutions protected from light (Figure 2). In fact, it is known that the presence of salts, organic matter along with metal salts with redox activity, such as Fe, Cu or Zn can affect the stability of hypochlorite solutions.
In the sample exposed to light (sample Nº 6), a sharp decrease of available chlorine concentration was observed, with a 3.6 % decrease at 24 h and 20.7 % at 48 h. These results confirm the importance of light protection to preserve bleach stability, whose BUD cannot be extended to more than 24 h when it is not protected from light. In line with this results, Rutala et al (20) informed a decrease in available chlorine concentration of 50 to 60 % after a 30 days storing period in transparent bottles.
These results are in agreement with those reported by Iqbal et al that recommended a maximum shelf-life of 4–6 days for bleach solutions of 0.5 g/L and 5 g/L, prepared with drinking water and maintained in inactinic containers at 20-35 °C (6). Other authors informed a longer BUD of, at least, 200 days for 5 g/L bleach solutions stored in the darkness at 24 °C (9). This increase in stability could be related to the use of sterile distilled water, where the presence of organic matter or other contaminants would be negligible.
Assigning the appropriate BUD to chlorine solutions is essential to quality control and to ensure the expected benefits of disinfection in both institutional and community settings. In our work, we have developed evidence-based recommendations to assign a BUD for 1 g/L bleach solutions prepared with drinking water. These conditions could be extrapolated to the household or hospital level, provided that clean and disinfected containers are used; and must be understood as a guideline to assign the BUD of bleach solutions used for disinfection purposes that could bring several benefits.
It is important to note that degradation can be accelerated under non-ideal conditions of preparation or storage, such as using contaminated water, storing solutions in open or non-opaque containers (20), or exposing them to high temperatures (21). Moreover, improper manipulation of the concentrated solutions or the more frequent opening of the containers could allow the external factors to have a more substantial influence in the degradation rate. In this context, the intervention of the Pharmacy Services is recommended, providing use protocols and training and supervising staff (10). Their involvement in determining active chlorine in both concentrated and diluted bleach solutions can also provide a greater certainty for its correct use in the health care settings.
7. Conclusions
The compliance of the available chlorine concentration in several trademark bleach solutions was confirmed. In addition, this study provides conclusive information on the period and storage conditions under which 1 g/L bleach solutions can be used as a surface disinfectant. Solutions were stable for 10 days when were stored at room temperature and in an opaque container. Instead, solutions stored without protection from light, only maintains its stability for 24 hours.
8. References
1. Ponzano GP. Sodium hypochlorite: History, properties, electrochemical production. Contrib Nephrol. 2007;154:7–23.
2. World Health Organization. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. WHO Guidel. 2014;1–156.
3. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–51.
4. Grupo de trabajo Farmacotecnia de la SEFH. RECOMENDACIONES SOBRE EL USO DE DESINFECTANTES Y LIMPIEZA EN LAS ÁREAS DE ELABORACIÓN DE MEDICAMENTOS FRENTE AL COVID 19 [Internet]. Madrid; 2020 [cited 2020 July 8]: [about 8 p.]. Available from: https://www.sefh.es/fichadjuntos/Desinfeccion-limpieza-Farmacotecnia-COVID19.pdf
5. Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006;11(4):147–57.
6. Iqbal Q, Lubeck-Schricker M, Wells E, Wolfe MK, Lantagne D. Shelf-life of chlorine solutions recommended in Ebola virus disease response. PLoS One. 2016;11(5):1–12.
7. Gordon G, Adam L, Bubnis B. Minimizing chlorate ion formation. J Am Water Works Assoc. 1995;87(6):97–106.
8. World Health Organization. Cleaning and disinfection of environmental surfaces in the context of COVID-19: interim guidance. Geneva, Switzerland; 2020.
9. Pişkin B & Türkün M. Stability of various sodium hypochlorite solutions. J Endod. 1995;21(5):253–5.
10. Aguas Lavandinas. DI-2019-7355-APN-ANMAT#MSYDS Argentina; (Sept 6, 2019) p. 1–6.
11. Johnson BR, Remeikis NA. Effective shelf-life of prepared sodium hypochlorite solution. J Endod. 1993;19(1):40–3.
12. Gélinas P, Goulet J. Heat and Light Stability of Eight Sanitizers. J Food Prot. 1982;45(13):1195–6.
13. Sirtes, G., Waltimo, T., Schaetzle, M., & Zehnder, M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod. 2005; 31(9), 669-6.
14. ABI. Detienen a tres personas en El Alto por envasar lavandina adulterada. Los Tiempos [Internet]. 2020 May 11[cited 2020 Jun 8]: [about 1 p.]. Available from: https://www.lostiempos.com/actualidad/pais/20200511/detienen-tres-personas-alto-envasar-lavandina-adulterada-ser-comercializada
15. Incautaron presunta lavandina adulterada de supermercados chinos. Noticias Las Flores [Internet]. 2020 May 2;1[cited 2020 Jun 8]: [about 1 p.]. Available from: https://www.noticiaslasflores.com.ar/69699/la-ciudad/incautaron-presunta-lavandina-adulterada-de-supermercados-chinos/?fb_comment_id=2978485532227019_2988464374562468
16. Docentes compararon la lavandina que entregaban en la escuela y el resultado fue sorprendente. RIO NEGRO [Internet]. 2020 Mar 13[cited 2020 Jun 8]: [about 2 p.]. Available from: https://www.rionegro.com.ar/docentes-custionaron-producctos-de-limpieza-entregados-por-el-gobierno-1286392/
17. 5220 CHEMICAL OXYGEN DEMAND (COD) (2017). In: Standard Methods For the Examination of Water and Wastewater. 23rd. 2018.
18. Chapman D, Kimstach V. Chapter 3: Selection of Water Quality Variables. In: Chapman D, editor. Water Quality Assessments- A Guide to Use of Biota, Sediments and Water in Environmental Monitoring. 2nd ed. UNESCO/WHO/UNEP; 1996.
19. Ministerio de Salud, Secretaría de Políticas Regulación e Institutos, Administración Nacional de Medicamentos Alimentos y Tecnología Médica, Instituto Nacional de Medicamentos. Farmacopea Argentina [Internet]. 7th ed. Comisión Permanente de la Farmacopea Argentina, editor. Farmacopea Argentina. Ciudad Autónoma de Buenos Aires; 2003 [cited 2020 Jun 8]: [2745 p.]. Available from: http://www.anmat.gov.ar/webanmat/fna/fna_pdfs.asp
20. Rutala WA, Cole EC, Thomann CA, Weber DJ. Stability and bactericidal activity of chlorine solutions. Infect Control Hosp Epidemiol. 1998 May;19(5):323-7. doi: 10.1086/647822
21. Nicoletti MA, Siqueira EL, Bombana AC, Oliveira GG. Shelf-life of a 2.5% sodium hypochlorite solution as determined by Arrhenius equation. Braz Dent J. 2009; 20(1): 27-31.
Acknowledgments
LCLG thanks CONICET for her postdoctoral fellowship. MEO is a member of CIC CONICET.