Page 104 - 83_02
P. 104
María José Gómez-Lechón Moliner
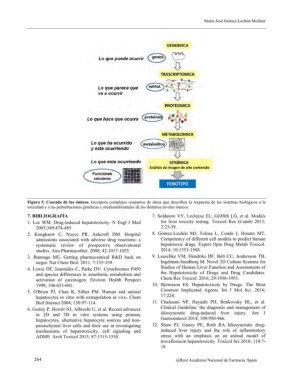
Figura 5. Cascada de las ómicas. Incorpora complejos conjuntos de datos que describen la respuesta de los sistemas biológicos a la
toxicidad y a las perturbaciones genéticas y medioambientales de los distintos niveles ómicos.
7. BIBLIOGRAFÍA 7. Soldatow VY, Lecluyse EL, Griffith LG, et al. Models
for liver toxicity testing. Toxicol Res (Camb) 2013;
1. Lee WM. Drug-induced hepatotoxicity. N Engl J Med 2:23-39.
2003;349:474-485.
8. Gómez-Lechón MJ, Tolosa L, Conde I, Donato MT.
2. Kongkaew C, Noyce PR, Ashcroft DM. Hospital Competency of different cell models to predict human
admissions associated with adverse drug reactions: a hepatotoxic drugs. Expert Opin Drug Metab Toxicol.
systematic review of prospective observational 2014; 10:1553-1568.
studies. Ann Pharmacother. 2008; 42:1017-1025.
9. Lauschke VM, Hendriks DF, Bell CC, Andersson TB,
3. Bunnage ME. Getting pharmaceutical R&D back on Ingelman-Sundberg M. Novel 3D Culture Systems for
target. Nat Chem Biol. 2011; 7:335-339. Studies of Human Liver Function and Assessments of
the Hepatotoxicity of Drugs and Drug Candidates.
4. Lewis DF, Ioannides C, Parke DV. Cytochromes P450 Chem Res Toxicol. 2016; 29:1936-1955.
and species differences in xenobiotic metabolism and
activation of carcinogen. Environ Health Perspect 10. Björnsson ES. Hepatotoxicity by Drugs: The Most
1998; 106:633-641. Common Implicated Agents. Int J Mol Sci. 2016;
17:224.
5. O'Brien PJ, Chan K, Silber PM. Human and animal
hepatocytes in vitro with extrapolation in vivo. Chem 11. Chalasani NP, Hayashi PH, Bonkovsky HL, et al.
Biol Interact 2004; 150:97-114. Clinical Guideline: the diagnosis and management of
idiosyncratic drug-induced liver injury. Am J
6. Godoy P, Hewitt NJ, Albrecht U, et al. Recent advances Gastroenterol 2014; 109:950-966.
in 2D and 3D in vitro systems using primary
hepatocytes, alternative hepatocyte sources and non- 12. Shaw PJ, Ganey PE, Roth RA Idiosyncratic drug-
parenchymal liver cells and their use in investigating induced liver injury and the role of inflammatory
mechanisms of hepatotoxicity, cell signaling and stress with an emphasis on an animal model of
ADME. Arch Toxicol 2013; 87:1315-1530. trovafloxacin hepatotoxicity. Toxicol Sci 2010; 118:7-
18
264 @Real Academia Nacional de Farmacia. Spain