Page 67 - 81_04
P. 67
Javier Martínez-Moreno et al.
4 10 20 Evolución?de?FVC?(%)
0
Medi a ?de? ca mbi o?FVC? (%) 30 40 50 60 70 MEDIA?PACIENTES
2 EC?PIPF -004/0 06
0 Semanas PLACEBO?PIPF-004/006
-2
-4
-6
-8
-10
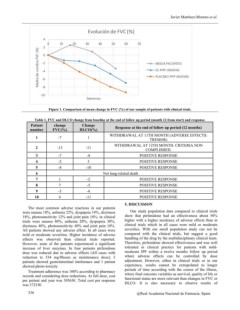
Figure 1. Comparison of mean change in FVC (%) of our sample of patients with clinical trials.
Table 1. FVC and DLCO change from baseline at the end of follow up period (month 12 from start) and response.
Patient change Change Response at the end of follow up period (12 months)
number FVC(%) DLCO(%)
1 -7 1 WITHDRAWAL AT 11TH MONTH (ADVERSE EFFECTS:
TREMOR)
2 -13 -11 WITHDRAWAL AT 12TH MONTH. CRITERIA NON
COMPLISHED.
3 -7 -8 POSITIVE RESPONSE
4 -2 3 POSITIVE RESPONSE
5 -8 -10 POSITIVE RESPONSE
6 Not lung-related death
71 -2 POSITIVE RESPONSE
87 -5 POSITIVE RESPONSE
9 -3 -6 POSITIVE RESPONSE
10 6 -11 POSITIVE RESPONSE
The most common adverse reactions in our patients 5. DISCUSSION
were nausea 18%, asthenia 22%, dyspepsia 19%, dizziness
18%, photosensitivity 12% and joint pain 10%; in clinical Our study population data compared to clinical trials
trials were nausea 40%, asthenia 20%, dyspepsia 30%, show that pirfenidone had an effectiveness about 50%
dizziness 40%, photosensitivity 40% and joint pain 10%. higher with a higher incidence of adverse effects than in
All patients showed any adverse effect. In all cases were clinical trials which in all cases were mild or moderate
mild or moderate severities. Higher incidence of adverse severities. With our small population study can not be
effects was observed than clinical trials reported. compared with the clinical trials, but suggest a good
However, none of the patients experienced a significant handling of the drug by the multidisciplinary clinical team.
increase of liver enzymes. In four patients pirfenidone Therefore, pirfenidone showed effectiveness and was well
dose was reduced due to adverse effects (All cases with tolerated in clinical practice for patients with mild-
reduction to 534 mg/8hours as maintenance dose); 3 moderate IPF within a twelve months follow up period
patients showed gastrointestinal intolerance and 1 patient where adverse effects can be controlled by dose
showed photo-toxicity. adjustment. However, either in clinical trials or in our
experience, results cannot be extrapolated to longer
Treatment adherence was 100% according to pharmacy periods of time according with the course of the illness,
records and considering dose reductions. At full dose, cost where final outcome variables as survival, quality of life or
per patient and year was 30563€. Total cost per response functional status are more relevant than changes in FVC or
was 37233€. DLCO. It is also necessary to observe results of
336 @Real Academia Nacional de Farmacia. Spain