Page 53 - 81_02
P. 53
Reactive oxygen species and vascular remodeling in cardiovascular diseases
AngII
AT1 MAPKs
ROS
Akt (ERK1/2, JNK,
Migration p38 MAPK)
NF-?B PKA
AP-1
Inmediate early genes
Fetal-type genes
Proliferation Hypertrophy
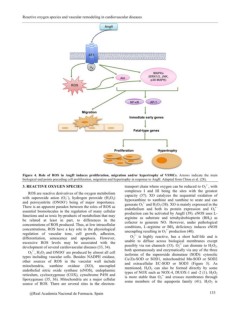
Figure 4. Role of ROS in AngII induces proliferation, migration and/or hypertrophy of VSMCs. Arrows indicate the main
biological end points preceding cell proliferation, migration and hypertrophy in response to AngII. Adapted from Chiou et al. (28).
3. REACTIVE OXYGEN SPECIES transport chain where oxygen can be reduced to O2•-, with
complexes I and III being the sites with the greatest
ROS are reactive derivatives of the oxygen metabolism capacity (37). XO catalyzes the sequential oxidation of
with superoxide anion (O2-.), hydrogen peroxide (H2O2) hypoxanthine to xanthine and xanthine to urate and can
and peroxynitrite (ONOO-) being of major importance. generate O2•- and H2O2 (38). XO is mainly expressed in the
There is an apparent paradox between the roles of ROS as endothelium and both its protein expression and O2•-
essential biomolecules in the regulation of many cellular production can be activated by AngII (39). eNOS uses L-
functions and as toxic by-products of metabolism that may arginine as substrate and tetrahydrobiopterin (BH4) as
be related at least in part, to differences in the cofactor to generate NO. However, under pathological
concentrations of ROS produced. Thus, at low intracellular conditions, L-arginine or BH4 deficiency induces eNOS
concentrations, ROS have a key role in the physiological uncoupling resulting in O2•- production (40).
regulation of vascular tone, cell growth, adhesion,
differentiation, senescence and apoptosis. However, O2•- is highly reactive, has a short half-life and is
excessive ROS levels may be associated with the unable to diffuse across biological membranes except
development of several cardiovascular diseases (33, 34). possibly via ion channels (33). O2•- can dismute to H2O2,
both spontaneously and enzymatically via any of the three
O2•-, H2O2 and ONOO- are produced by almost all cell isoforms of the superoxide dismutase (SOD): cytosolic
types including vascular cells. Besides NADPH oxidase, Cu/Zn-SOD or SOD1, mitochondrial Mn-SOD or SOD2
other sources of ROS in the vascular wall include and extracellular EC-SOD or SOD3 (Figure 5). As
mitochondria, xanthine oxidase (XO), uncoupled mentioned, H2O2 can also be formed directly by some
endothelial nitric oxide synthase (eNOS), endoplasmic types of NOX such as NOX-4, DUOX-1 and -2 (1). H2O2
reticulum, cyclooxygenase (COX), cytochrome P450 and is more stable than O2•- and crosses membranes through
lipoxygenase (35, 36). Mitochondria are a major cellular some members of the aquaporin family (41). H2O2 is
source of ROS. There are several sites in the electron-
@Real Academia Nacional de Farmacia. Spain 133