Page 56 - 84_02
P. 56
The chromenopyrazole scaffold in the modulation of the endocannabinoid system: a broad therapeutic prospect
Figure 7. Some examples of quinoid compounds with antitumor activity.
Since cancer is a complex multifactorial disease, in a single molecule (58–60). In this context, ortho and
successful treatment of neoplasms often requires para cannabinoid-quinones were recently reported in the
pharmaceutical intervention at multiple pathways. This is literature (61–64). They were designed using the
generally accomplished by using a combination of chromenopyrazole scaffold as structural core
different drugs. However, a very attractive and promising (chromenopyrazolediones, figure 8).
approach is to target different anticancer modes of action
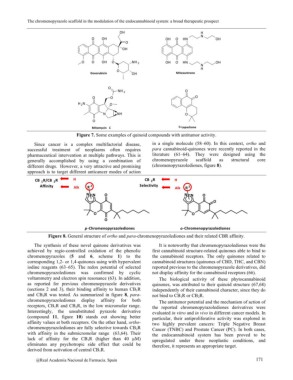
Figure 8. General structure of ortho and para-chromenopyrazolediones and their related CBR affinity.
The synthesis of these novel quinone derivatives was It is noteworthy that chromenopyrazolediones were the
achieved by regio-controlled oxidation of the phenolic first cannabinoid structure-related quinones able to bind to
chromenopyrazoles (5 and 6, scheme 1) to the the cannabinoid receptors. The only quinones related to
corresponding 1,2- or 1,4-quinones using with hypervalent cannabinoid structures (quinones of CBD, THC, and CBN)
iodine reagents (63–65). The redox potential of selected reported previous to the chromenopyrazole derivatives, did
chromenopyrazolediones was confirmed by cyclic not display affinity for the cannabinoid receptors (66).
voltammetry and electron spin resonance (63). In addition,
as reported for previous chromenopyrazole derivatives The biological activity of these phytocannabinoid
(sections 2 and 3), their binding affinity to human CB1R quinones, was attributed to their quinoid structure (67,68)
and CB2R was tested. As summarized in figure 8, para- independently of their cannabinoid character, since they do
chromenopyrazolediones display affinity for both not bind to CB1R or CB2R.
receptors, CB1R and CB2R, in the low micromolar range.
Interestingly, the unsubstituted pyrazole derivative The antitumor potential and the mechanism of action of
(compound 11, figure 10) stands out showing better the reported chromenopyrazolediones derivatives were
affinity values at both receptors. On the other hand, ortho- evaluated in vitro and in vivo in different cancer models. In
chromenopyrazolediones are fully selective towards CB2R particular, their antiproliferative activity was explored in
with affinity in the submicromolar range (63,64). Their two highly prevalent cancers: Triple Negative Breast
lack of affinity for the CB1R (higher than 40 µM) Cancer (TNBC) and Prostate Cancer (PC). In both cases,
eliminates any psychotropic side effect that could be the endocannabinoid system has been proved to be
derived from activation of central CB1R. upregulated under these neoplastic conditions, and
therefore, it represents an appropriate target.
@Real Academia Nacional de Farmacia. Spain 171