Page 61 - 84_02
P. 61
Paula Morales, Pilar Goya, Nadine Jagerovic
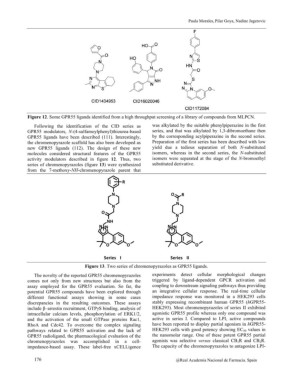
Figure 12. Some GPR55 ligands identified from a high throughput screening of a library of compounds from MLPCN.
Following the identification of the CID series as was alkylated by the suitable phenylpiperazine in the first
GPR55 modulators, N-(4-sulfamoylphenyl)thiourea-based series, and that was alkylated by 1,3-dibromoethane then
GPR55 ligands have been described (111). Interestingly, by the corresponding acylpiperazine in the second series.
the chromenopyrazole scaffold has also been developed as Preparation of the first series has been described with low
new GPR55 ligands (112). The design of these new yield due a tedious separation of both N-substituted
molecules considered structural features of the GPR55 isomers, whereas in the second series, the N-substituted
activity modulators described in figure 12. Thus, two isomers were separated at the stage of the N-bromoethyl
series of chromenopyrazoles (figure 13) were synthesized substituted derivative.
from the 7-methoxy-NH-chromenopyrazole parent that
Figure 13. Two series of chromenopyrazoles as GPR55 ligands.
The novelty of the reported GPR55 chromenopyrazoles experiments detect cellular morphological changes
comes not only from new structures but also from the triggered by ligand-dependent GPCR activation and
assay employed for the GPR55 evaluation. So far, the coupling to downstream signaling pathways thus providing
potential GPR55 compounds have been explored through an integrative cellular response. The real-time cellular
different functional assays showing in some cases impedance response was monitored in a HEK293 cells
discrepancies in the resulting outcomes. These assays stably expressing recombinant human GPR55 (hGPR55-
include ß–arrestin recruitment, GTP?S binding, analysis of HEK293). Most chromenopyrazoles of series II exhibited
intracellular calcium levels, phosphorylation of ERK1/2, agonistic GPR55 profile whereas only one compound was
and the activation of the small GTPase proteins Rac1, active in series I. Compared to LPI, active compounds
RhoA and Cdc42. To overcome the complex signaling have been reported to display partial agonism in hGPR55-
pathways related to GPR55 activation and the lack of HEK293 cells with good potency showing EC50 values in
GPR55 radioligand, the pharmacological evaluation of the the nanomolar range. One of these potent GPR55 partial
chromenopyrazoles was accomplished in a cell- agonists was selective versus classical CB1R and CB2R.
impedance-based assay. These label-free xCELLigence The capacity of the chromenopyrazoles to antagonize LPI-
176 @Real Academia Nacional de Farmacia. Spain