Page 54 - 84_02
P. 54
The chromenopyrazole scaffold in the modulation of the endocannabinoid system: a broad therapeutic prospect
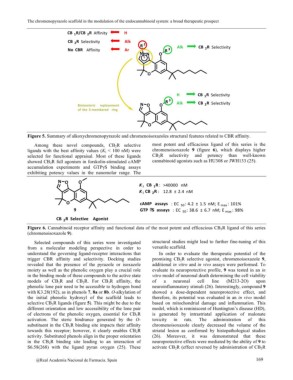
Figure 5. Summary of alkoxychromenopyrazole and chromenoisoxazoles structural features related to CBR affinity.
Among these novel compounds, CB2R selective most potent and efficacious ligand of this series is the
ligands with the best affinity values (Ki < 100 nM) were chromenoisoxazole 9 (figure 6), which displays higher
selected for functional appraisal. Most of these ligands CB2R selectivity and potency than well-known
showed CB2R full agonism in forskolin-stimulated cAMP cannabinoid agonists such as HU308 or JWH133 (25).
accumulation experiments and GTP?S binding assays
exhibiting potency values in the nanomolar range. The
Figure 6. Cannabinoid receptor affinity and functional data of the most potent and efficacious CB2R ligand of this series
(chromenoisoxazole 9).
Selected compounds of this series were investigated structural studies might lead to further fine-tuning of this
from a molecular modeling perspective in order to versatile scaffold.
understand the governing ligand-receptor interactions that
trigger CBR affinity and selectivity. Docking studies In order to evaluate the therapeutic potential of the
revealed that the presence of the pyrazole or isoxazole promising CB2R selective agonist, chromenoisoxazole 9,
moiety as well as the phenolic oxygen play a crucial role additional in vitro and in vivo assays were performed. To
in the binding mode of these compounds to the active state evaluate its neuroprotective profile, 9 was tested in an in
models of CB1R and CB2R. For CB1R affinity, the vitro model of neuronal death determining the cell viability
phenolic lone pair need to be accessible to hydrogen bond of a neuronal cell line (M213-2O) upon
with K3.28(192), as in phenols 7, 8a or 8b. O-alkylation of neuroinflammatory stimuli (26). Interestingly, compound 9
the initial phenolic hydroxyl of the scaffold leads to showed a dose-dependent neuroprotective effect, and
selective CB2R ligands (figure 5). This might be due to the therefore, its potential was evaluated in an in vivo model
different orientation and low accessibility of the lone pair based on mitochondrial damage and inflammation. This
of electrons of the phenolic oxygen, essential for CB1R model, which is reminiscent of Huntington’s disease (HD),
activation. The steric hindrance generated by the O- is generated by intrastriatal application of malonate
substituent in the CB1R binding site impacts their affinity toxicity in rats. The administration of this
towards this receptor; however, it clearly enables CB2R chromenoisoxazole clearly decreased the volume of the
activity. Substituted phenols align in the proper orientation striatal lesion as confirmed by histopathological studies
in the CB2R binding site leading to an interaction of (26). Moreover, it was demonstrated that these
S6.58(268) with the ligand pyran oxygen (25). These neuroprotective effects were mediated by the ability of 9 to
activate CB2R (effect reversed by administration of CB2R
@Real Academia Nacional de Farmacia. Spain 169