Page 51 - 84_02
P. 51
Paula Morales, Pilar Goya, Nadine Jagerovic
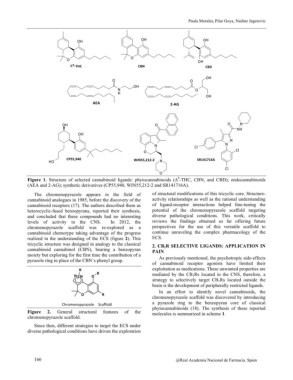
Figure 1. Structure of selected cannabinoid ligands: phytocannabinoids (?9-THC, CBN, and CBD); endocannabinoids
(AEA and 2-AG); synthetic derivatives (CP55,940, WIN55,212-2 and SR141716A).
The chromenopyrazole appears in the field of of structural modifications of this tricyclic core. Structure-
cannabinoid analogues in 1985, before the discovery of the activity relationships as well as the rational understanding
cannabinoid receptors (17). The authors described them as of ligand-receptor interactions helped fine-tuning the
heterocyclic-fused benzopyrans, reported their synthesis, potential of the chromenopyrazole scaffold targeting
and concluded that these compounds had no interesting diverse pathological conditions. This work, critically
levels of activity in the CNS. In 2012, the reviews the findings obtained so far offering future
chromenopyrazole scaffold was re-explored as a perspectives for the use of this versatile scaffold to
cannabinoid chemotype taking advantage of the progress continue unraveling the complex pharmacology of the
realized in the understanding of the ECS (figure 2). This ECS.
tricyclic structure was designed in analogy to the classical
cannabinoid cannabinol (CBN), bearing a benzopyran 2. CB1R SELECTIVE LIGANDS: APPLICATION IN
moiety but exploring for the first time the contribution of a PAIN
pyrazole ring in place of the CBN´s phenyl group.
As previously mentioned, the psychotropic side-effects
Figure 2. General structural features of the of cannabinoid receptor agonists have limited their
chromenopyrazole scaffold. exploitation as medications. These unwanted properties are
mediated by the CB1Rs located in the CNS, therefore, a
strategy to selectively target CB1Rs located outside the
brain is the development of peripherally restricted ligands.
In an effort to identify novel cannabinoids, the
chromenopyrazole scaffold was discovered by introducing
a pyrazole ring to the benzopyran core of classical
phytocannabinoids (18). The synthesis of these reported
molecules is summarized in scheme 1.
Since then, different strategies to target the ECS under
diverse pathological conditions have driven the exploration
166 @Real Academia Nacional de Farmacia. Spain