Page 129 - 88_01
P. 129
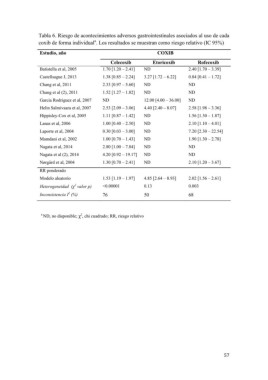
Tabla 6. Riesgo de acontecimientos adversos gastrointestinales asociados al uso de cada
coxib de forma individuala. Los resultados se muestran como riesgo relativo (IC 95%)
Estudio, año COXIB
Batistella et al, 2005 Celecoxib Etoricoxib Rofecoxib
Castellsague J, 2013
Chang et al, 2011 1.70 [1.20 – 2.41] ND 2.40 [1.70 – 3.39]
Chang et al (2), 2011 1.38 [0.85 – 2.24] 3.27 [1.72 – 6.22] 0.84 [0.41 – 1.72]
Garcia Rodriguez et al, 2007 2.33 [0.97 – 5.60] ND
Helin Salmivaara et al, 2007 1.52 [1.27 – 1.82] ND ND
Hippisley-Cox et al, 2005 12.00 [4.00 – 36.00]
Lanas et al, 2006 ND 4.40 [2.40 – 8.07] ND
Laporte et al, 2004 2.53 [2.09 – 3.06] ND
Mamdani et al, 2002 1.11 [0.87 – 1.42] ND ND
Nagata et al, 2014 1.00 [0.40 – 2.50] ND 2.58 [1.98 – 3.36]
Nagata et al (2), 2014 0.30 [0.03 – 3.00] ND 1.56 [1.30 – 1.87]
Nørga° rd et al, 2004 1.00 [0.70 – 1.43] ND 2.10 [1.10 – 4.01]
RR ponderado 2.80 [1.00 – 7.84] ND 7.20 [2.30 – 22.54]
Modelo aleatorio 4.20 [0.92 – 19.17] ND 1.90 [1.30 – 2.78]
1.30 [0.70 – 2.41]
Heterogeneidad (?2 valor p) ND
Inconsistencia I2 (%) ND
2.10 [1.20 – 3.67]
1.53 [1.19 – 1.97] 4.85 [2.64 – 8.93] 2.02 [1.56 – 2.61]
<0.00001 0.13 0.003
76 50 68
a ND, no disponible; ?2, chi cuadrado; RR, riesgo relativo
57